OSE-230 was developed to activate a unique mechanism for resolving chronic inflammation, focusing on modulation of macrophages and neutrophils.

OSE-230 was developed to activate a unique mechanism for resolving chronic inflammation, focusing on modulation of macrophages and neutrophils.

The trial reportedly showcased significant weight reduction in those treated with VK2735, a dual GLP-1/GIP receptor agonist.

Agency identified several clinical deficiencies in the study, including insufficient evidence for roluperidone in the treatment of the negative symptoms associated with schizophrenia.
New label marks the first Bruton’s tyrosine kinase inhibitor to be approved with an oral suspension formulation.
Results of the study determined that adult flu vaccination rates among Medicaid populations were significantly low, highlighting other health inequities.

In an interview with Pharm Exec Associate Editor Don Tracy, Arun Krishna, VP, Head of US Lung Cancer Franchise, AstraZeneca, talks about the FDA's approval of TAGRISSO with the addition of chemotherapy in adult patients with locally advanced or metastatic epidermal growth factor receptor-mutated non-small cell lung cancer.

In the EAGLE-1 Phase III trial, gepotidacin met the primary efficacy endpoint of non-inferiorty to the current leading treatment for uncomplicated urogenital gonorrhea.
Collaboration aims to leverage Nhwa’s expertise in the country’s neuro-psychiatric health sector.

Results of a study conducted by the National Institutes of Health indicate that the Paxlovid prevented a substantial number of hospitalizations associated with COVID-19.

Funds expected to advance multiple programs into clinical studies, including FMC-376, which targets KRASG12C cancers.

Letter emphasizes the need to protect children from potentially harmful medical advice spread on social media.

Early-stage trial results indicate that NLRP3 inflammasome inhibitors were able to achieve nearly the same weight loss as Wegovy while also reducing inflammatory biomarkers linked to heart disease.

TEV-’574 has shown potential as a treatment for inflammatory bowel diseases such as ulcerative colitis and Crohn disease.
Researchers highlight several benefits and concerns associated with the launch of direct-to-consumer models for GLP-1 receptor agonists.
Amtagvi is the first one-time, individualized T-cell therapy approved by the FDA for any solid tumor cancer.
Positive Phase III study results for Tonmya (cyclobenzaprine HCl sublingual tablets) will be basis of New Drug Application to the FDA for the management of fibromyalgia.

In an interview with Pharm Exec Associate Editor Don Tracy, Raj Verma, Chief Diversity, Culture, and Experience Officer, Sanofi, discusses features of the company's support program for patients diagnosed with cancer and other critical illnesses.

Investigators harness machine learning to evaluate response to sertraline treatment for major depressive disorder.
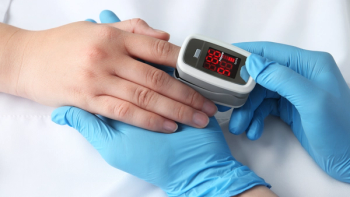
The MightySat Fingertip Pulse Oximeter is reportedly the first pulse oximeter available without a prescription.
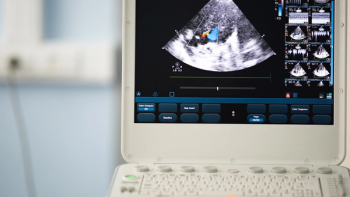
Device designed to address tricuspid regurgitation through a vein in the leg.

Treatment aims to reduce the risk of finger or toe amputation due to frostbite.
Joint venture aims to submit an Investigational New Drug application to the FDA by early next year for treatment that targets solid tumors.

In an interview with Pharm Exec Associate Editor Don Tracy, Jonathan Miller, General Counsel, US Hemp Roundtable, discusses recent efforts that urge the House Energy and Commerce Committee to expedite an FDA hearing on the hemp market.
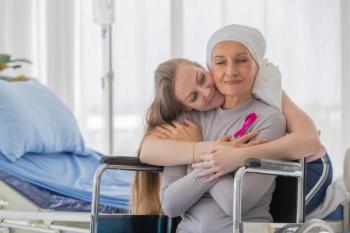
Cancer & Work: Acting Together guarantees job security and full salary continuation for up to twelve months for Sanofi employees and family members diagnosed with critical illnesses.
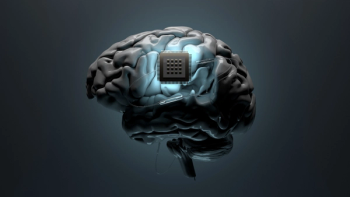
Synchron’s brain-computer interface device is being developed to allow patients with mobility challenges to operate certain technology with their mind.
Higher prices of insulin attributed to significant annual increases, outpacing general inflation.

In an interview with Pharm Exec Associate Editor Don Tracy, Shubh Goel, Head of Immuno-Oncology and Gastrointestinal Tumors, US Oncology Business Unit, AstraZeneca, provides her thoughts on the success of the trial.
The US Department of Health and Human Services has initiated a comprehensive strategy to address the escalating syphilis rates, as a result.

DELFI-Tumor Fraction assay was developed to improve noninvasive assessment of tumor burden and monitoring of treatment efficacy and resistance in patients with advanced cancers.
Rusfertide is currently in a pivotal Phase III clinical trial as a potential first-in-class treatment for polycythemia vera.