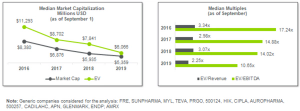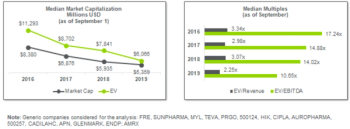
505(b)(2): A Pathway to Competitiveness Through Innovation for Specialty and Generic Companies
Published: | Updated:
As the market for specialty and generic products continues to become more competitive and pricing pressures increase, the 505(b)(2) pathway may allow companies more options to diversify their portfolios.