
We all make mistakes — and that includes those involved in Health Technology Assessment (HTA). Following an error made by ICER this year, Leela Barham looks at similar mistakes made in the past and what can be done about them.
Leela Barham is a freelance health economist and policy expert. She has published in peer-reviewed journals and presented at national and international conferences. She has provided advice to the Department of Health and Social Care on policy on pricing of branded medicines to inform the negotiation of a successor to the UK’s Pharmaceutical Price Regulation Scheme (PPRS), the Voluntary Scheme for Branded Medicines Pricing and Access (VPAS), as well as worked with patient groups, the NHS, pharmaceutical companies and many others internationally on the economics of healthcare and pharmaceuticals. Contact Leela on leels@btinternet.com

We all make mistakes — and that includes those involved in Health Technology Assessment (HTA). Following an error made by ICER this year, Leela Barham looks at similar mistakes made in the past and what can be done about them.

Leela Barham talks to Yvette Venable, the Institute for Clinical Effectiveness Review's new Vice President of Patient Engagement, about how ICER is ramping up its efforts to bring patient organizations along on its journey.

ISPOR has published its third issue of the top trends in HEOR. Leela Barham takes a look at how the list has changed over time.

Leela Barham talks to Dr. Brian O’Rourke, outgoing President and Chief Executive Officer of the Canadian Agency for Drugs and Technologies in Health (CADTH), about his 11 in years in the role.

Leela Barham reacts to the Institute for Economic and Clinical Review's very first Unsupported Price Increase (UPI) report.

Leela Barham talks to Sir David Haslam, the outgoing Chair of UK’s National Institute for Health and Care Excellence (NICE), about the highs and lows of his six-year tenure at the organization.

The UK has had in place what amount to two pricing schemes for branded medicines for years. But with major changes to both schemes set out in 2018, it’s now a new tale of two pricing schemes. Leela Barham reports.

Among the new acronyms watch is UPI, or unjustified price increase, recently coined by ICER as part of its new workstream to explore price increases for both branded and generic drugs in the US.

The Institute for Clinical Effectiveness Review (ICER) has set up a working group to bring together the expertise of NICE and other key HTAs to develop new methods to guide value-based pricing of potential cures. Leela Barham reports.

President Trump's International Pricing Index proposals amount to international reference pricing (IRP), something that countries in Europe have been doing for years. Leela Barham asks, What lessons can be learned from there?
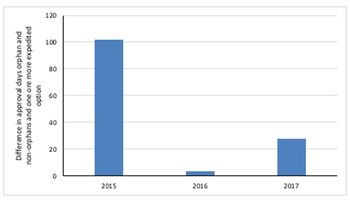
Leela Barham looks at whether there is a boost to the speed of FDA approval when a drug not only secures one or more of the FDA’s expedited development and review methods, but also when they are also designated an orphan drug.
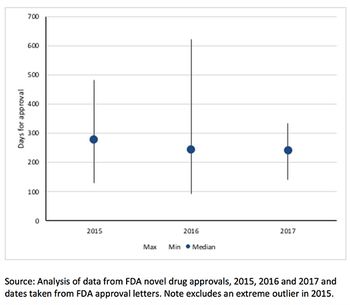
There’s been a lot of talk about speeding up drug approval at FDA. So just how fast can approval be? Leela Barham takes a look at the speed of approval for each of FDA's expedited development and review methods.
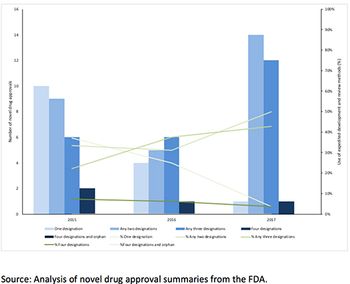
Leela Barham takes a look at the take-up of the existing options to speed up FDA approval.



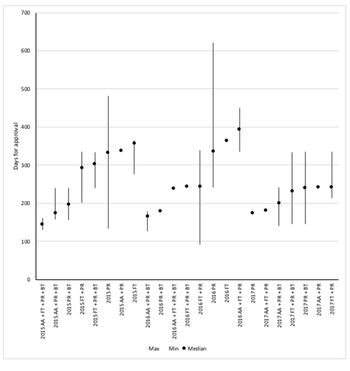
FDA offers four options to speed up approval and they can be used in combination. But just which of the many combinations offers the fastest approval? Leela Barham reports.


Leela Barham reviews the Institute for Clinical Effectiveness Review's announced pilot program, which allow manufacturers to scrutinize executable versions of draft cost-effectiveness models.

Professor Carole Longson's move from NICE to the ABPI is another appointment that helps to square the balance between UK industry and Government. Leela Barham reports.

The UK's NICE now has the job of both being a member of the newly named Accelerated Access Collaborative (AAC) and also acting as its Secretariat.

A year after the Accelerated Access Review explored how to speed up adoption of innovation across the NHS in England, the UK Government has responded. Leela Barham reports.

Leela Barham looks at the reasons behind the Association of the British Pharmaceutical Industry's bid to legally challenge NICE's proposed introduction of the budget impact test.

Leela Barham casts her eye over the UK's plans for its life sciences industry in the post-Brexit world.

With a new consultation from the UK’s Department of Health proposing changes to the Statutory Scheme for Pricing of Branded Medicines, what could this mean for negotiation of a successor to the 2014 Pharmaceutical Price Regulation Scheme (PPRS)? Leela Barham reports.

Everyone’s talking value, but not everyone is willing to pin down the elusive concept. Pharm Exec talks with Steven Pearson, founder of the Institute for Clinical Effectiveness Review (ICER), about all things value-based.

The UK is entering into unchartered waters between Brexit – offering potentially new approaches to procurement – and negotiating a successor to the PPRS. The government's new recruit Steve Oldfield could give it the inside track, writes Leela Barham.

Tensions are becoming clear within industry as it faces what might yet be the toughest negotiations on pricing with the UK government. Leela Barham reports.


Value frameworks are now "sexy". In the US, no fewer than five have emerged since around 2015. Leela Barham discusses how the value assessment landscape is evolving.

Published: October 1st 2014 | Updated:

Published: October 27th 2014 | Updated:

Published: September 30th 2014 | Updated:

Published: September 29th 2014 | Updated:

Published: September 2nd 2014 | Updated:

Published: July 8th 2014 | Updated: