
The UK's proposed Accelerated Access Review is being dogged by delay and disappointment, writes Leela Barham.
Leela Barham is a freelance health economist and policy expert. She has published in peer-reviewed journals and presented at national and international conferences. She has provided advice to the Department of Health and Social Care on policy on pricing of branded medicines to inform the negotiation of a successor to the UK’s Pharmaceutical Price Regulation Scheme (PPRS), the Voluntary Scheme for Branded Medicines Pricing and Access (VPAS), as well as worked with patient groups, the NHS, pharmaceutical companies and many others internationally on the economics of healthcare and pharmaceuticals. Contact Leela on leels@btinternet.com

The UK's proposed Accelerated Access Review is being dogged by delay and disappointment, writes Leela Barham.

The Association of the British Pharmaceutical Industry is seeking permission for a Judicial Review of NICE's affordability proposals. Leela Barham reports.
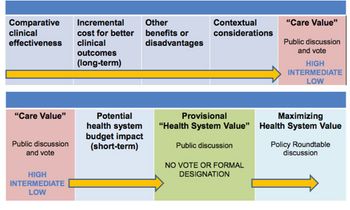
With the US Institute for Clinical and Economic Review (ICER) looking like it is here to stay, Leela Barham considers how its value framework is evolving.
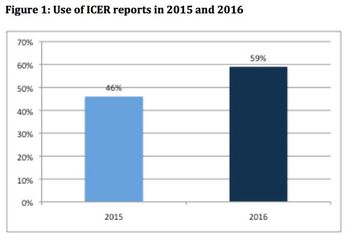
In the second of her articles on US value assessment, Leela Barham reviews the emergence of multiple frameworks and looks at their potential impact.

No one thought Brexit would be easy and in the wake of last week's UK election the going has only gotten tougher. Leela Barham looks at the fallout from a life sciences perspective.

With the UK's General Election set for June 8, Leela Barham looks what the competing parties are promising for pharma and healthcare

The Innovation Scorecard was developed to track compliance with NICE Technology Appraisals. Leela Barham reviews its latest update.
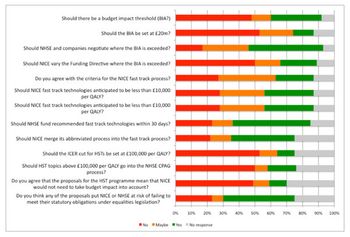
NICE and NHSE have been grappling with the issue of affordability. It’s not a new challenge, but with the advent of new treatments that are both cost effective and unaffordable, something has to be done. Leela Barham reports.

Leela Barham looks at NICE's new position statement, which seeks to set out a little more clearly how it works with industry.

The Lancet's series of papers on moving from universal health care coverage to "right care" for health offers a truly global and comprehensive perspective, writes Leela Barham. But just what does "right care for health" mean for the industry?

Leela Barham speaks to NICE International and Imperial College’s Institute of Global Health Innovation about joining forces under the International Decision Support Initiative.

That may be one option as the UK agency, like the NIH it supports, looks at how to generate big savings.

The UK's NICE, like the NHS it supports, is looking at how to make big savings. One option is to get more "commercial". Leela Barham takes a look.

Leela Barham looks at the 18 recommendations of England’s Accelerated Access Review (AAR), which proposes how to speed up adoption of the best innovation in the NHS.

The UK is moving closer to aligning its two approaches to regulating drug pricing - the voluntary PPRS and statutory price cuts - but the headaches are likely to continue, writes Leela Barham.

Leela Barham looks at the potential cost to industry of the National Institute for Health and Care Excellence's plans to charge for TAs.

Leela Barham reviews NHS England's proposals for the creation of Regional Medicines Optimisation Committees.

If managed access is to be a widely available option, it makes sense to re-think just how much should be spent on it, writes Leela Barham.

A look at the EU plans for a European patent, and where the UK fits in the mix.


Leela Barham looks at the EU plans for a European patent, and asks where the UK will fit in after Brexit.

NHS England head Simon Stevens believes the UK's PPRS needs significantly updating.

Is a more "light-touch" appraisal process the key to getting the UK's NICE to do more with less? Leela Barham reports.


NICE's notion to charge the industry for its technology appraisals looks set to become reality, writes Leela Barham.

Following the consultation on the future of England's Cancer Drug Fund, Leela Barham reviews what the stakeholders are thinking.

February 17, 2016.

Will the UK learn from past efforts to champion innovation in the NHS? Leela Barham assesses the Phase 1 evaluation of its Innovation, Health and Wealth initiative.

November 25, 2015.

The much awaited consultation on a ‘new’ Cancer Drugs Fund (CDF) in England has finally emerged. Leela Barham reports.