
In the patent scrum, a litigator finds her true self.

In the patent scrum, a litigator finds her true self.

Whatever used to be wrong with the world of big Pharma could be fixed with a single tag phrase: emerging country markets. Most of the majors have invested heavily in this geographic segment, and the biggest of the big-companies like Novartis-now rely on it for more than a quarter of their global sales. Like all good things, however, there are shadows amidst the sunlight, and the task of turning volume sales into sustainable profits is getting harder.

Canada’s Rx&D, the industry’s oldest functioning trade association, recently changed its name-with a mission of bridging the many points of collaboration that drive the process of modern drugs, from discovery to market.

A closer reading of this year's JP Morgan investor conference identifies three areas where the insular, often maladroit tone of the industry-investor dialogue may be morphing to something more grounded in the larger societal context of healthcare.

Ferring Pharmaceuticals’ new manufacturing facility in Parsippany, NJ.

A Pharm Exec conversation with AmerisourceBergen CEO Steve Collis

A new discipline-population health-contests disease treatment as an end in itself. It asks: besides advancing the science of medicine, what do biopharmaceuticals actually do to improve health overall?

Building on our annual look at the industry's product pipeline, four key themes stand out that are driving the fast-changing environment for drug development.

Driven by knowledge advances and the importance of a more collaborative commercial model, intellectual property (IP) has morphed from an arcane, specialized function dominated by technicians to a showcase strategic priority of the “c-suite.” This trend is hardly restricted to the life sciences. In fact, some of the most creative work in using IP tools to extract more value from knowledge assets is taking place among businesses that operate across multiple sectors.

AmerisourceBergen believes the power of community begins with talk that gets the blood flowing.

A new and more compelling strategy must be crafted to engage critics on drug pricing and bolster biopharmaceuticals as a leader in technological innovations that lower health costs overall.

A new IMS study reveals how changes in the design of commercial/employer-based health insurance plans are accelerating the push to higher patient contributions to the cost of prescribed drugs. William Looney reports.

A closer look at our Industry Audit numbers hints to a clear divide between two alternative strategies for long-term growth: scale versus focus.

Experts from industry and academia probe the changing role of the pharmacoepidemiology function as it grapples with the challenges of big data analytics, tightening drug safety requirements, growing post-approval study burdens, and payer expectations around the application of real-world evidence.

The Tufts Center for the Study of Drug Development aims to plug the knowledge gap at the lower end of China's trade business through a partnership with Shanghai’s Fudan University.

In a knowledge industry like biopharma, people are the intangible asset that marks the difference between a status quo or standout performance. Pharm Exec recently convened a panel of industry team leaders and HR specialists at St. Joseph’s University Haub School of Business to parse out some useful best practices in three key areas: talent recruitment and retention; skills training; and workforce diversity.

Amid the release of our eighth list of Emerging Pharma Leaders, it's fitting to take a look at the trends and values that will determine true leadership in big Pharma in the years ahead.

William Looney reviews EvaluatePharma's World Preview 2015 - Outlook to 2020, launched at last week's BIO International Convention.

Institutionalizing the teachable moment with GENiE, and new network focused on advancing the idea that for healthcare to change, the education of its leaders must change first.

Pharm Exec talks to Matt Wallach, President of Veeva Systems, an upstart start-up whose mix of cutting-edge technology and strong customer orientation has brought it to the top rank in providing cloud-based data management services to the industry.

William Looney reports on last week's Global Educators Network for Health Care Innovation Education meeting, focused on shared learning and best practices to drive reforms in both the public and private sectors.
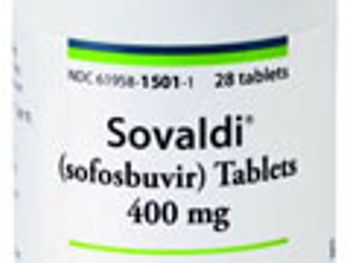
Pharm Exec’s 9th annual Brand of the Year selection is Gilead Science’s back-to-back cures for HCV. We profile how both drugs reversed expectations around the listless product launch and revived industry reputation for startling breakthrough innovations.

Gilead outlines its key access initiatives for its hepatitis C franchise.

What’s new in the growing field of global market access?

Expert panel on rare disease highlights the challenges of serving expectant patients in this uniquely complex market access environment.

William Looney speaks to Dr. Claudia Graeve, who leads the Healthcare Businesswomen's Association's sole international chapter in Europe, on improving the prospects for female managers in the region.

Learnings gleaned from our annual EAB meeting, hosted this year by Dr. David Nash, Dean of the Thomas Jefferson University School of Population Health.

Pharm Exec's Emerging Pharma Leaders 2013

Pharma jumps on board personal genomics train-at least for test drive-but will journey ultimately help transform treatment or stall out as just another fad?

South Africa may have the most developed market for biopharmaceuticals on the African continent, but there are a few critical shortcomings, writes William Looney.