Articles by Guest Blogger

The National Institute for Health and Care Excellence (NICE) will have a vital role in the post 2014 value-based pricing (VBP) world. The Department of Health (DH), which still acts as the sponsor organization for the agency, has set out in more detail just what value assessment by NICE will need to do. Now NICE has to determine the methods and set up the governance and workable processes to deliver it.

Recently, there was a bit of a dust-up over whether it was appropriate for the Secretary of Health and Human Services (HHS) to engage the National Football League (NFL) to help HHS with the process of drumming-up enrollment for health insurance exchanges. In the end, the NFL and other sports leagues decided they were not going to be involved fearing the appearance of taking political sides.

Joe Ganley, Director of State Government Affairs at McKesson, spoke earlier this year on public health policy and its implications for patient adherence. Here, he provides PharmExec with some highlights of that presentation.

So, you’ve done it. You have left big business or forsaken the dusty halls of academia to launch that start-up of your own.

In the wake of the meningitis outbreak caused by contaminated compounded medications produced by the New England Compounding Center (NECC).

With the Presidential election decided and President Obama the clear winner, it’s time for Pharma to get down to business on the implementation of ObamaCare.

This year has been marked by flat to declining growth rates, but there are hopeful signs for 2013, writes Andrew Forman.

Pharmaceutical industry leaders Novartis and Merck-among others-released second-quarter 2012 results showing global sales down in third-quarter 2012, with growth in key pharmaceutical products helping to offset losses due to patent expirations.

The sudden departure of EC Health Commissioner John Dalli tops an already difficult year for the European pharma regulators, writes Reflector.

No doubt the November 6th election is going to be close, very close: close for the Presidency, close for the Senate, and possibly close for the House.

Just days before the reports that R&D investment in Spanish pharma had fallen to below EUR1 billion ($1.3 billion) in 2011 for the first time since 2007.

Ethics is a matter of trust, which in the regulatory space requires transparency and openness among all stakeholders, including patients and industry.

New stories earlier this year (1) reported that FDA scientists have been suspected of leaking confidential, commercial, and trade secret information to the media.

With the Supreme Court’s decision in June to uphold the Patient Protection and Affordable Care Act (ACA), life science companies gained access to 32 million potential new customers.

The furore that burst across Brussels in late September over reported findings of tumors in rats fed with genetically modified maize may seem only tangentially related to the concerns.

A recent decision by the Department of Health (DoH) to introduce a compliance regime for NICE (National Institute for Health and Clinical Excellence) technology.
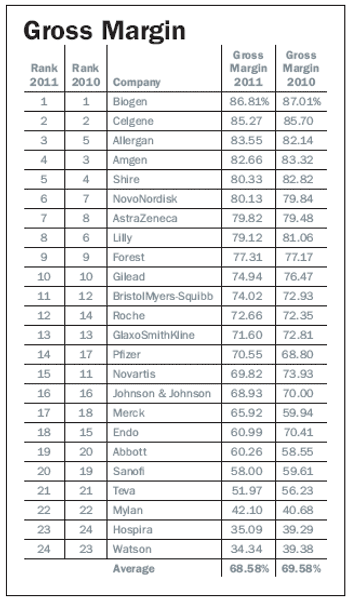
Pharm Exec’s 2012 Industry Audit relies on 2011 data to compare performance against 2010.

The Access to Medicines Index is an attempt to measure and compare the corporate social responsibility of both innovator (20) and generics (7) companies based on a number of different (and often quixotic) indicators.

The FDA and the Advanced Medical Technology Association (AdvaMed) gave cardiovascular device manufacturers plenty to think about over the holiday weekend with information presented at an Aug. 28 workshop.

Public policy activities should be a distinct, mission critical function for Big Pharma - our business depends increasingly on government as a key customer.

The European Union is at last groping its way towards some form of standardization in judging the value of medicines.

The European Union is at last groping its way towards some form of standardization in judging the value of medicines.

Well, we certainly have a presidential campaign now, don’t we? Good grief. Since Rep. Paul Ryan was named the Republican VP candidate last week.

Patient groups are a growing force, not just in the debate around tactical areas of medical specialities, but also on the vital question of pricing and reimbursement rules. But will their voices be heard? Reflector reports.

It may surprise some working in the industry, but pharmaceutical companies, in particular those in Europe, rank among the world’s best online corporate communicators, writes David Bowen.

Last month, Pharm Exec, the Tufts Center for the Study of Drug Development and software vendor Clear Trial brought companies together for an editorial exchange on current challenges in budgeting and spend for clinical trials.

EFPIA president Andrew Witty is hoping that the deepening European economic crisis will impel European leaders towards more courageous and radical solutions, writes Reflector.

Patients who live for many years following a cancer diagnosis will likely require additional support, and a whole new kind of follow-up care.

Will France’s new socialist president, François Hollande, prove too strong for pharma’s taste? Reflector, Pharm Exec’s EU correspondent, reports.

The business outlook for pharma companies for the rest of 2012 and beyond is mixed, as pharma companies struggle to realign themselves to a new business model that will work.




