
In the last three decades, MNCs have contributed greatly to the transfer of knowledge to generic companies in areas such as technology,

In the last three decades, MNCs have contributed greatly to the transfer of knowledge to generic companies in areas such as technology,

Every day, your competitors are doing everything within their power to steal your customers.

There has been a lot of concern about the decision to give the National Institute for Health and Care Excellence (NICE) the responsibility to look at “highly specialized technologies” (HSTs, or orphan drugs to you and me).

Global price management will lead the fight-back on margins, writes Arnaud Grunwald.

The European Medicines Agency thought it might at last be back on the road to salvation when Guido Rasi swept into town in 2011 and started ordering greater transparency in the agency’s operations.

Human behavior may be the most significant variable in the future of cancer care in two sense.

At this year’s Bio IT World Conference, Thomas Verish, Group Director of Data Operations Services at Bristol-Myers Squibb,

In light of the risks and challenges inherent in the development of oncologics.

It’s hardly news that biopharmaceutical companies today face increasing pressure to innovate, produce, improve efficiency and quality.

As expected, the PFE/AZ saga has just begun to play out, and as we listen to the music of mega-mergers once again.

The Pfizer-AstraZeneca courtship raises many fascinating questions about the future shape of the international pharmaceutical industry.

A CCG had denied funding for oocyte preservation for a woman with Crohn’s disease before she had bone marrow transplant and chemotherapy.

The dizzying flurry of press releases from both Pfizer Inc. and AstraZeneca over a possible PFE acquisition of AZN have Rx experts on both sides of the Atlantic buzzing.

New data maintenance guidelines have sparked a flurry of activity in Europe.

The Incremental Cost Effectiveness Ratio (ICER) thresholds used by agencies such as the UK’s National Institute for Health and Care Excellence (NICE).
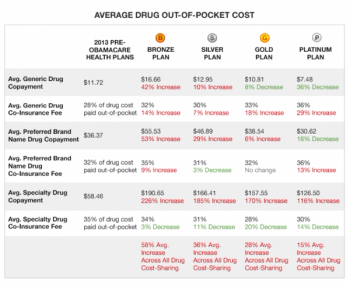
In the midst of the hue and cry last week over the public release of individual Medicare payments to physicians, I was surprised to read that one of Medicare’s most costly medical services is - ophthalmology.

Overall, last year can be considered quite a successful one in terms of new product approvals.

Health IT is going through an era of both expansion and convergence, in which the universe of available information is growing exponentially.

We spend a lot of time focusing on what our advertising says to physicians, what our reps say to physicians, and what our websites and various other channels communicate to patients.

The European Medicines Agency is determined to minimize opposition to its next moves on releasing clinical trial data.

Pharma has operated the same way for 100 years, but the US healthcare model has changed completely in the last 30 years, for payers, pharmacists, hospitals, and even at the clinician level.

It was recently said, in reference to the UK’s Value-Based Assessment(VBA) program, that innovation had not been “tried and tested in the pricing context”.

Few would argue with the desirability of innovation - something new, something that delivers value.

The new Internet Library from the European Patients’ Academy (EUPATI) will come online towards the end of 2015.

Patient adherence to medicine is the eternal puzzle for big Pharma: what combinations of financial incentives, personalized interventions, and just good information work best to push down that stubborn 50 per cent abandonment threshold after six months of treatment?

Recent Congressional investigations over the pricing scheme of Gilead’s much-heralded hepatitis C drug Sovaldi

In January 2013, FDA told manufacturers to lower the dose of zolpidem, the insomnia treatment, for women, and suggested a 10 mg to 5 mg for immediate-release products (Ambien, Edluar, and Zolpimist) and from 12.5 mg to 6.25 mg for extended-release products (Ambien CR).

Peter Houston looks at the pharma marketing takeaways from last week’s Digital Innovation Summit.

The National Institute for Health and Care Excellence (NICE) has finally published the formal consultation on value-based assessment (VBA).
With the introduction of every new technology, tactic, or gimmick comes what I call a tactical scrum. It’s like watching young kids play their first game of T-ball or soccer.