Articles by Guest Blogger

As Europe departs for its annual holiday-sacrosanct, even amid the continuing economic woes, the multiple crises in the Middle East, and the dark mysteries of Russia’s intentions in its former satellites-the question remains unresolved as to what to expect from the new European Parliament in the new term.

Over the years, healthcare professionals have needed to adapt to a more highly informed patient population due to improvements in the availability of accessible healthcare information through the internet.

With all of the buzz and excitement around risk-based monitoring (RBM), one might expect that the broad adoption of this emerging clinical research paradigm is well underway.

The European Union’s struggle to bring its data protection rules into the 21st century continues-haltingly-as the EU itself enters its new era, with a new European Parliament, a new President-designate of the European Commission, and a new European Commission due to take office in November.

It is well known that Brazil has an immense biodiversity and that the Amazon is the largest tropical rainforest in the world.

The European Medicines Agency (EMA) has insisted that the concerns raised about its scientific advice given to pharmaceutical companies stem from a flawed understanding of the activities of the agency and its partners in this area.

The medical technology industry has a legacy of creating life-enhancing, life-extending products while rewarding its investors in the process.

EvalutePharma’s seventh edition of its World Preview documents that worldwide prescription drug sales forecast will exceed the one trillion dollar mark by 2020.

Life sciences and biotech research clusters across the UK, including those linked to university research departments are benefiting from an improved funding climate and ongoing consolidation in the pharmaceutical sector.

Recruiting patients remains one of the most difficult challenges clinical trial sponsors face. Recognizing that fewer than five percent of eligible adult patients enroll in therapeutic trials.

Patients are influential in the UK when it comes to access to medicines: their individual stories are highlighted in the media and the groups that represent them are credited with major policies.

The Drug Information Association (DIA), one of the largest non-profit organizations supporting clinical research and drug development, held its 2014 annual meeting in San Diego last week. Applied Clinical Trials’ Moe Alsumidaie looks at three of the key themes from this year’s meeting: incorporating advocacy groups and patient voices in clinical research, breakthrough research applications, and new data collection methodologies.

Andy Bender and Geert van Gansewinkel outline eight lessons for executing a soft change program for enhancing compliance effectiveness within European life sciences organizations.

For pharma companies, entering the social media scene is not as simple as just creating a Facebook page or learning how to tweet in 140 characters or less.
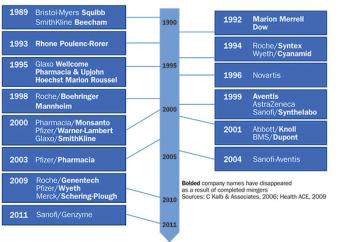
The major pressures hindering pharmaceutical industry success have not changed-payer constraints on drug costs, R&D productivity.

Brunei is harnessing its rich biodiversity and the growing halal market in a bid to develop its pharmaceutical sector.

The recent geopolitical developments in Ukraine have seriously influenced the conditions and the environment for clinical trials in Ukraine and in Russia.
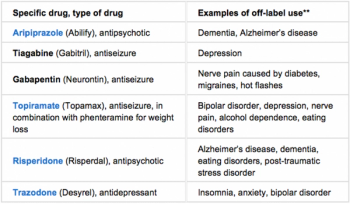
Years ago, while sitting in an Rx company lunch room, I was listening to one of the company’s top sales reps discuss the commercial progress a recently launched prescription was making.

Speaking at the 2014 New York BIO conference, FDA Commissioner, Dr. Margaret Hamburg, addressed the pressing issues affecting the biopharmaceutical industry and the FDA

Among the many announcements Apple made earlier this month at its annual World Wide Developer’s Conference, one was of particular interest to the healthcare industry.

The UK’s National Institute for Health and Care Excellence (NICE) set out their proposals for value-based assessment (VBA) in March 2014.

While at the 2014 New York BIO Conference (NYBIO), Applied Clinical Trials‘ Moe Alsumidaie spoke to Nathan Tinker, Executive Director at the New York Biotechnology Association.

There is more collaboration than ever between payers and providers in the drive to provide high-quality.

One of EMA’s key objectives this year is improvements in dealing with the “causes and impact of shortages of human medicines caused by GMP non-compliance and quality defects”.

A European bid to impose additional limits on research involving human embryos has been defeated.

Effective clinical trial management depends on accurate and unbiased performance measurement.

FDA, in partnership with other federal and international agencies, has taken action against websites that sell potentially dangerous, unapproved prescription drugs to US consumers.
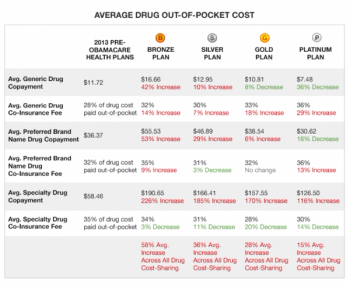
We are now approaching the six month point in the implementation of the new Obamacare program. It’s senseless to go into the good, bad and ugly of this new program.

In today’s pharmaceutical industry, it seems there are nearly as many communication channels as there are instruments in the Philharmonic.

Technology offers the promise of engaging patients on a level never seen before.








