
FDA may be receiving fewer new drug applications for truly innovative products, but it has been overwhelmed this year with 800 abbreviated NDAs for generic drugs and thousands of supplements.
Jill Wechsler is Pharm Exec's Washington Corespondent

FDA may be receiving fewer new drug applications for truly innovative products, but it has been overwhelmed this year with 800 abbreviated NDAs for generic drugs and thousands of supplements.

The circumstances of Crawford's departure may complicate the process of securing a permanent FDA leader. Congress and HHS are investigating whether his confirmation process failed to uncover important facts.

Many still regard FDA's approach as costly and unnecessary. But the prospect of qualifying for purchase by PEPFAR is attracting applicants. The policy has helped bring news AIDS treatments to market.

FDA could lose 251 employees under the administration's proposed 2006 budget. The agency will try to do more with less through risk-management practices, but important tasks will fall by the wayside.

Integra v. Merck KGaA supports research by large pharmaceutical companies, but it also opens the door to greater use of compounded materials by all parties. Congress may have to clarify its scope.
Pharma companies believe that they can compete in an e-prescribing environment if information systems permit full disclosure and allow doctors to create bookmarks that link easily to information they want.

CMS envisions studies to show which drugs keep patients out of hospitals or how certain treatments can reduce side effects. Such analysis would support decisions on best practices in using medications.

Just a decade ago, FDA was accused of dragging its feet on new drug applications. Now, supposedly, the agency is moving so fast that it's letting unsafe, insufficiently tested products into the marketplace.

Reformers want to give FDA more clout, but agency officials say internal changes will help fix drug safety problems.

Should policy makers expect 90 percent of seniors to enroll in PDPs, or will 75 percent be enough? Will the program have to keep costs down to $400 billion a year, or will spending be linked to savings elsewhere?

One response to today's safety concerns is particularly alarming: The research community has become skittish about conducting clinical studies involving pain medications and other high-risk treatments.

Expanded coverage for the uninsured and Medicaid reform are low priorities at the White House.

Finger pointing has begun in earnest, as Congress tries to decide who ignored what warning, who silenced what staff scientist, and who broke what law. But recent events also raise real long-term issues for industry.

Waxman promised to put teeth in the proposal by levying fines on sponsors of clinical trials that fail to comply.

Tremendous changes are underway at FDA headquarters in Rockville, Maryland. Whole sections of biologics review moving from one center to another. Regulatory activities being refocused on risk. Restructured roles for agency field inspectors. And a variety of other reforms, many driven by the specter of bioterror. Despite operating for almost two years without a commissioner, FDA is moving full speed ahead with a major reorganization and a new regulatory philosophy.

After months of speculation, in September the White House finally nominated its lead health policy advisor, Mark McClellan, as the next FDA commissioner. McClellan is a physician and economist and, most recently, a member of the White House Council of Economic Advisors. As an MD with no direct ties to the pharmaceutical industry, he fits the basic criteria set for confirmation by the Democratic-controlled Senate.

Multi-tier formularies that set higher co-pays for the most expensive pharmaceuticals reduce beneficiaries' overall spending on prescriptions.

FDA's September announcement that it plans to transfer some therapeutic biologic review functions from the Center for Biologics Evaluation and Research (CBER) to the Center for Drug Evaluation and Research (CDER) caught most agency officials and Washington observers by surprise.

Consumers who lack pharmacy benefit coverage are more likely to ask physicians about the cost of treatments than those who have coverage, according to data from FDA research on the impact of direct-to-consumer advertising.
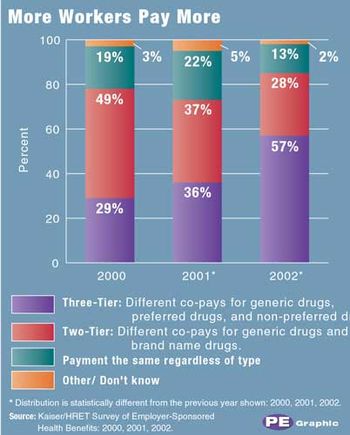
In an effort to cope with soaring healthcare costs, more employers are establishing three-tier prescription drug benefit plans for workers.

Acting on the belief that states should respect agency rulings involving labeling and advertising, FDA filed legal briefs challenging judges that second-guess its authority.

Instead of developing innovative therapies, pharma companies devote most of their resources to modifying existing medications, according to a report from the National Institute for Health Care Management Foundation (NIHCM).

The House leadership collected just enough votes in late June to approve a Medicare reform bill that provides some coverage of pharmaceuticals for seniors. Democrats rebuked the measure as a scam and a fraud; some conservatives complained that it opened the door to a costly new entitlement.

In a closely watched patent case that has important implications for pharma companies, the Supreme Court ruled unanimously in May to uphold policies that protect patent holders from imitators. The decision, in what is considered one of the most significant patent disputes to come before the court, is expected to benefit brand-name companies that bring patent infringement cases against generics makers.

As part of an aggressive campaign against the rising cost of medicines, AARP, the national advocacy group for Americans over 50, joined three class-action lawsuits against pharma companies involving alleged anticompetitive efforts to block generic competition and inflate prices.
Does FDA harm patients by rushing unsafe products to market or by slowing product development and approval? That debate came to the fore recently with the almost simultaneous release of competing studies.

First Amendment protection of commercial speech got a big boost last month when the US Supreme Court ruled 5-4 that FDA's policy to limit advertising for pharmaceutical compounding is unconstitutional.
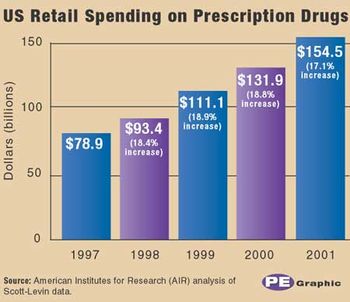
The National Institute of Health Care Management made headlines with its report on double-digit increases (17 percent) in retail spending on medicines in 2001. The total reached $155 billion last year, almost double the $80 billion spent in 1997, according to the study, "Another Year of Escalating Costs." PhRMA president Alan Holmer said the increase is a good thing, signifying that more people who need medicines for chronic conditions are being treated and thereby avoiding more expensive medical procedures.

African-American physicians regard direct-to-consumer advertising of prescription medicines as one way to educate minority patients about needed treatment and healthcare options, according to a survey conducted by the National Medical Association (NMA). Almost all of the 900 physicians answering the questionnaire reported that DTC advertising has prompted patients to ask questions, and one-third acknowledged that they feel additional pressure to justify their prescribing decisions. But almost half (48 percent) said that such promotion increased communications between physicians and patients.

Among the dozens of initiatives in Washington affecting the pharma industry, one common thread is the growing movement to control accelerating expenditures for prescription medicines.