
Approval of Kerendia was based on results from the Phase III FINEARTS-HF trial, which demonstrated statistically significant and clinically meaningful reductions in cardiovascular events across a broad range of patients with heart failure.


Approval of Kerendia was based on results from the Phase III FINEARTS-HF trial, which demonstrated statistically significant and clinically meaningful reductions in cardiovascular events across a broad range of patients with heart failure.
The new drug application is supported by 48-week data from the Phase III MK-8591A-051 and MK-8591A-052 trials, which showed that the doravirine/islatravir combination was non-inferior to both baseline antiretroviral therapy and to bictegravir/emtricitabine/tenofovir alafenamide in treating HIV.
Approval was based on results from the Phase IIIb TRAILBLAZER-ALZ 6 trial, which showed that Kisunla achieved comparable efficacy in amyloid plaque and P-tau217 reduction while lowering the risk of amyloid-related imaging abnormalities with edema or effusion in patients with early Alzheimer disease.
The full approval of Spikevax covers children aged six months through 11 years who are at increased risk for severe COVID-19, transitioning the vaccine from emergency use to full licensure.
The drug is currently in Phase 1 clinical trials for gastric cancer treatments.
Approval was based on results from the DOORwaY90 trial, which demonstrated a 98.5% overall response rate and 100% local tumor control in patients treated with the SIR-Spheres Y-90 resin microspheres for unresectable hepatocellular carcinoma.
Ekterly becomes the first FDA-approved oral on-demand treatment for hereditary angioedema in patients aged 12 years and older.
Accelerated approval was based on results from the Phase I/II LINKER-MM1 trial, which showed a 70% objective response rate in patients with relapsed or refractory multiple myeloma treated with Lynozyfic.
Results from the Phase III ZENITH trial show that patients treated with Winrevair (sotatercept-csrk) for pulmonary arterial hypertension experienced a 76% reduction in the composite risk of death, lung transplant, and ≥24-hour hospitalization.
Updated label allows Neuraceq to be used in selecting patients for amyloid-targeting therapies and includes the use of quantitative PET imaging metrics to support diagnosis and monitor disease progression in Alzheimer’s care.
The supplemental premarket approval application is supported by Phase III data, which showed significant improvements in neck appearance in patients treated with Skinvive.
Gamifant marks the first-ever FDA-approved treatment for both adult and pediatric patients with hemophagocytic lymphohistiocytosis macrophage activation syndrome in the context of known or suspected Still's disease.
Label changes remove Risk Evaluation and Mitigation Strategy programs and eases monitoring requirements, supporting broader access to Bristol Myers Squibb’s CAR T-cell therapies Breyanzi and Abecma for patients with large B cell lymphoma and multiple myeloma.

The label update for Amyvid adds guidance for assessing amyloid plaque levels and may help inform diagnostic and treatment decisions in patients being evaluated for Alzheimer disease.
Approval was based on results from the Phase II TROPION-Lung05 trial, which showed that Datroway demonstrated a strong overall response rate and duration of response in patients with previously treated, locally advanced or metastatic EGFR-mutated non-small cell lung cancer.
Approval was based on results from the Phase III inMIND trial, which demonstrated a statistically significant and clinically meaningful improvement in progression-free survival in patients treated with Monjuvi combined with rituximab and lenalidomide for relapsed or refractory follicular lymphoma.

Dupixent is the first and only targeted therapy to receive FDA approval for bullous pemphigoid.

Harliku becomes the first FDA-approved treatment for alkaptonuria, indicated to reduce homogentisic acid levels in affected adults.
Approval of first twice-yearly HIV pre-exposure prophylaxis was based on results from the Phase III PURPOSE 1 and PURPOSE 2 trials, which show that ≥99.9% of patients treated with Yeztugo remained HIV-negative.

The investigational MRI contrast agent, gadoquatrane, is designed to deliver effective imaging at a significantly reduced gadolinium dose, supporting safer repeat use in adults and pediatric patients.

Under the new initiative, companies may receive a voucher enabling FDA review to be shortened from the standard 10–12 months to just 1–2 months following final application submission if the drug addresses US national health priorities.

Andembry is the first prophylactic therapy for hereditary angioedema to target factor XIIa, inhibiting the top of the inflammatory cascade that drives attacks.
mRESVIA is now authorized for use in adults aged 18–59 years with an increased risk of respiratory syncytial virus-related lower respiratory tract disease.
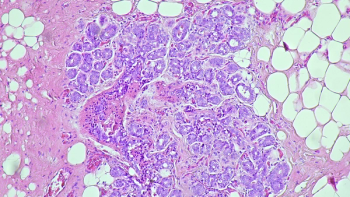
Keytruda marks the first perioperative anti-PD-1 treatment option for adults with PD-L1–positive resectable locally advanced head and neck squamous cell carcinoma.
Approval of Zusduri was based on data from the Phase III ENVISION trial, which showed a 78% complete response at three months in patients with recurrent low-grade, intermediate-risk non-muscle invasive bladder cancer.