
For pharma marketers, the good news is recent advances in social media monitoring make it possible to listen to or engage with patients on social media websites.

For pharma marketers, the good news is recent advances in social media monitoring make it possible to listen to or engage with patients on social media websites.

Successful innovation now has to align with key metrics of value-can an old baseball metaphor help guide the way?

Pricing and personalized medicine are key themes shaping drug development and marketing

Let's Modernize Post-Approval Regulation of Prescription Drugs

High prices, murky financial relations, and a reluctance to disclose clinical data undermine public trust in industry and the research enterprise.

The US and Europe are moving toward the enactment of new legislation that will change the way pharma products are handled and shipped throughout the supply chain.

The Sunshine Act's Open Payments spending disclosure program is now live. The government's lead compliance officer explains how it will work.

The ?70 billion budget is still subject to final agreement on the European Union's overall spending plans for 2014-2020.

The US and EU move forward with measures to fortify the pharmaceutical supply chain, writes Patricia Van Arnum.

FDA wants pharma leaders to do more to ensure drug quality at home and abroad. Our Washington correspondent Jill Wechsler reports.
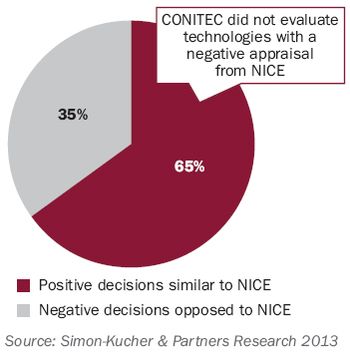
What is really driving decision making by the new Brazilian HTA agency? A look at appraisals issued in the first year.

Compounders are under increased scrutiny following last year's spinal meningitis outbreak.

While not as bleak as believed, the outlook of European biotech sector is struggling compared with its US counterpart.

Opioid abuse generates calls for efforts to curb distribution, develop abuse-resistant formulations.
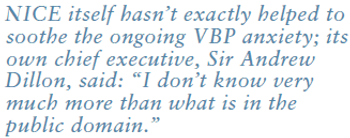
Value-based pricing in the United Kingdom is getting closer, but it remains out of focus.

Legal battles and regulatory missteps undermine access to low-cost generics, at home and abroad, writes Jill Wechsler.

The European Union's attempt to update its transparency rules sparks new debate.

There seems no end to demands for data on clinical research, conflicts of interest, company payments, and drug prices.

European Commision blocks the authorization of a life-saving liver drug outside of France.

After a decade of strife, the dialogue between industry and government appears to have entered a positive new phase. Julian Upton reports.

Compliance officers have risen into management's highest ranks, by choice in some organizations, and by government decree in others. Either way, their importance as a strategic partner can hardly be understated.
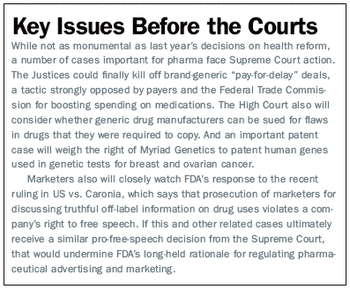
FDA policies will reshape drug development, while pressure to cut healthcare spending may alter drug coverage and pricing.

If people can promote drugs for uses that lack supporting evidence, it would turn back the clock to the pre-1962 world of medicine where there wasn’t any research or data on what medicines worked and what was harmful, says Robert Temple, deputy director for clinical science at FDA’s Center for Drug Evaluation and Research (CDER).

Increased scrutiny and new regulation have seen pharma’s relationship with key opinion leaders (KOLs) undergo a paradigm shift in recent years.

Tax and budget decisions will shape the healthcare market and drug research and regulation.