
Josh Reisberg outlines the foundation of a broad, overall defense strategy for generic companies embroiled in Hatch-Waxman patent infringement litigations.

Josh Reisberg outlines the foundation of a broad, overall defense strategy for generic companies embroiled in Hatch-Waxman patent infringement litigations.

Jonathan Kraft shares his family's secrets on what it takes to lead a successful organization, while also creating lasting brand power.

If companies are serious about building a sustainable organization for their employees and becoming a partner to key accounts, they need to be prepared to remodel their whole business around it, writes Miranda Wheatley Price.

With so much information readily available companies have lost a significant level of control over how and when details about their progress in drug development and planning related to approval, launch, and commercialization are disseminated.

The role of the medical affairs professional in the biopharma hierarchy has grown considerably in recent years, but challenges remain in its accent to true strategic partner with the C-suite.

Mark Bouch, Stephen Bungay, and David Roblin outline how the technique of "mission command", a military approach to setting direction and executing strategy in the pharmaceutical industry, has developed over the last ten years.

Before entering into risk-sharing agreements, manufacturers must understand the challenges and implications, writes Kimberly E. White.

Pamela Buffone from Privacy Analytics discusses growth in the specialty pharma sector and making patient data protection a top priority.
Jayachandra Reddy and Rishit Thakkar discuss the challenges facing early entrants in the Non-alcoholic Steatohepatitis market.

With the NASH pipeline filling up fast, Mariel Metcalfe looks at what pharma companies need to do to ensure their products do not end up as also-rans in this dynamic new market segment.

Our latest review reveals that those companies scoring well on profit management metrics are best positioned to maintain that crucial edge in performance execution.
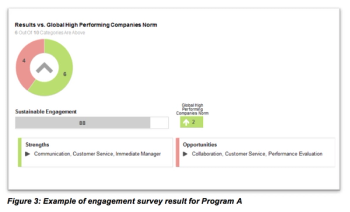
A review of a recent FSP in which the CRO partner assumed clinical monitoring and site support responsibility for more than 50 ongoing trials.

Following the unveiling of the UK’s post-Brexit life sciences strategy, Neil Grubert looks at the measures related to market access, a particular challenge in the UK market and key to its future success in the sector.
As regulators lower evidentiary requirements for approval to speed development and review of new drugs for unmet medical needs, payers are demanding more data to justify price premiums. Companies need to be strategic in how they navigate these complexities, write Bengt Anell, Sangeeta Budhia and Richard Macaulay.

Experts assess the current pharma dealmaking landscape-one where, despite market headwinds, is still heavily influenced by factors under the buyer and seller’s control.

In the first of three blogs on how digital strategy and a “data culture” can help define and deliver value, David Ormesher focuses on Value Creation.

Big data has proven to be a valuable business asset, but using it to gain competitive advantage requires the right combination of strategy, technology and execution, write Mahmood Majeed, Vickye Jain, and Sandeep Varma.

With the patient voice growing louder in all aspects of the drug development and commercialization journey, Pharm Exec examines some of the current industry thinking on the evolving pharma-patient relationship.
Jackie DeAngelis outlines three common mistakes that hinder access attainment when launching a new drug.

For pharma, diagnosing what ails or strengthens performance in this critical corporate discipline may help reshape business prognosis.

Study examines why a pharma brand’s early sales are not a trusted predictor for total lifetime value-it’s about driving waves of growth.

Pharm Exec's Editorial Advisory Board member, Peter Young, cover a summary of the strategic issues facing the biopharma industry, but goes on to tell the story of what happened last year and this first quarter in terms of the stock market, M&A and financing (including IPOs) activity, where it is headed, and the implications for senior management.

Pharm Exec convenes a panel of biopharma executives responsible for the Latin America business to discuss investment, market access, and reimbursement issues in this key and challengingly diverse growth market for the life sciences industry

Global market access analyst Neil Grubert discusses what are likely to be some of the key trends in market access this year and beyond, and the implications for the life sciences industry.

Simon Webster looks at the role of intellectual property (IP) management in protecting pharmaceutical company products.