
Assessing the landscape of the pharma industry’s readiness for future pandemics by looking at past pandemics, funding and revenue, and sustainability.

Assessing the landscape of the pharma industry’s readiness for future pandemics by looking at past pandemics, funding and revenue, and sustainability.

Addressing the challenges faced by Global Medical Affairs teams and how they can find balance to create maximum value for their organizations.

In this article, Eli Lilly & Co. examines risk management in the context of partnerships with other companies.

Dr. George Westerman talks to Michael Wong about how smarter executives will successfully lead their workforces in a post COVID-19 business environment.

Beyond necessity and nice-to-have, embracing real-world evidence as a true strategic differentiator.

While COVID-19 has shifted some of the rules, research reveals that practice changes were already afoot for market newcomers.

Uncovering fish oil derivative’s benefit for heart health.

New hope accompanies overdue recognition in sickle cell disease fight.
The world’s most expensive drug put in context.

How adoption of the key account management (KAM) model can help drive pharma’s agility in reaching and influencing a more diverse set of healthcare customers and decision-makers.

Amid pandemic, rely on the fundamentals.

Looking at four building blocks of success, Wolfram Lux and Simone Seiter investigate what it takes to win and be successful when commercializing an orphan drug

A look at how pharmaceutical company, Paratek is navigating the launch of a new antibiotic during the COVID-19 pandemic.

While HCPs are turning away from in-person meetings with sales reps amidst the COVID-19 pandemic, there are still strategies for them to keep their pipelines full.

Dr. Manuel Hermosilla shares his thoughts on how the pharmaceutical industry reacts to challenges that arise during trials, specifically during the search for an effective COVID-19 vaccine.

How pharma leaders can convert data collected for compliance purposes from a cost center into a critical success factor.
In adjusting to the long-term side-effects of the coronavirus pandemic, pharma companies will need to focus on change in three areas. Ben van der Schaaf and Aurelien Guichard report.

Immunomedics’ CFO/CBO Usama Malik discusses how a lot of good planning and a little good fortune helped the company to achieve its “virtual launch” amid the coronavirus crisis.

Amid the limitations of traditional compliance programs that emphasize control measures, here are steps biopharma companies can implement in their own cultures to ensure they’re behaving and performing with integrity.
A recent roundtable of pharmaceutical CEOs, and moderated by Dr. Scott Gottlieb, discussed pressing concerns around the pandemic, including clinical trials, product launches, keeping employees safe and the future of the pharmaceutical industry.

While COVID-19 has shifted the rules of the healthcare industry, research shows there was already progress on changes being made for how pharma companies are launching products.

With the role of Medical Information in pharma companies changing in recent years, this article outlines some current and future trends including globalization and the use of data to drive change.
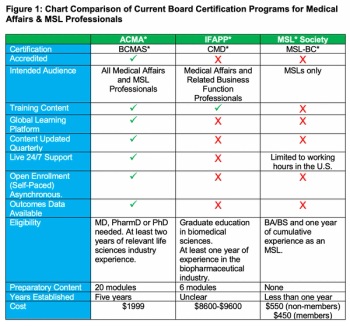
With the growth of the medical science liaison (MSL) role in mind, this article provides a comprehensive review of the board certification programs that are currently available to MSLs and other medical affairs professionals.
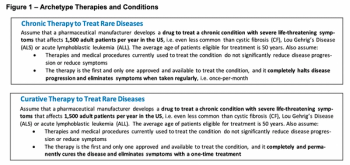
There has been a considerable amount of attention given to the rising costs of pharmaceutical and biotechnology therapies in the US. In early 2020, Charles River Associates conducted this research to determine what "fair" prices would be for these therapies.
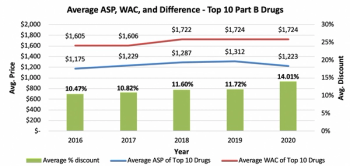
While there has been an increase in discourse concerning drug pricing transparency in recent years, there is still a degree of misunderstanding. This is due to the healthcare industry's numerous stakeholders and their compelling interests. This research done by Guidehouse aims to clear the picture.