US-based Bayer Healthcare Pharmaceuticals, as its executives were quick to point out, has now received three new approvals for cancer indications in the last eight months

US-based Bayer Healthcare Pharmaceuticals, as its executives were quick to point out, has now received three new approvals for cancer indications in the last eight months

During the Rutgers Business School’s annual healthcare symposium, an FDA official encouraged industry to put its drugs on the reviewing table and be prepared for good news.

A night of comedy in support of a chronic disease.

The Office of Prescription Drug Promotion (OPDP) is reorganizing its two primary advertising review divisions – professional and consumer – in an attempt to stay in line with the silo-breaking, multi-channel promotional output pouring in from pharma.

If a patient or physician encounters pharma-created content that doesn’t pop with relevance or utility, it doesn’t matter how multitudinous the channels are that carry it.

In 2012, Merck's diabetes franchise became the highest-selling product family in its 122-year history.

During his fifth State of the Union address, President Obama struck many familiar chords – including a line about ending certain subsidies to pharmaceutical companies – and emphasized, repeatedly, the need to get things done on the policy front.

Good science can’t be rushed, even when the lives of patients hang in the balance. But regulatory science, and its relationship to a drug’s commercial success or failure

After years of asking questions, the National Highway Traffic Safety Administration (NHTSA)is moving closer to a protocol "for assessing the driving-impairment risks of drugs". Ben Comer reports.

At CBI’s 10th Annual Pharmaceutical Compliance Congress, Maame Ewusi-Mensah Frimpong, deputy assistant attorney general, consumer protection branch, civil division, at the US Department of Justice, said being compliant means understanding people, and their motivations.
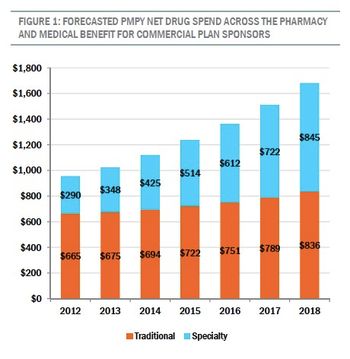
By 2018, the amount health insurers spend per person on specialty, or biologic drugs, will equal or surpass the amount spent on traditional products, according to a new report.

On average, cancer docs in the US would much rather deal with pharma sales reps in person as opposed to online, and drug companies are hiring more reps to support a slew of newly approved oncologic drugs.

The perennial fat got chewed during the first two days at J.P. Morgan’s annual healthcare conference – buy back stock or raise dividends? – and the usual suspects lurked, but newcomers like Walgreens and not-so-pharma companies like Life Technologies packed the presentation halls and corridors,

Participants in the 23rd Annual NYPF General Assembly last Friday outlined the many challenges facing industry, and the importance of collaboration as a way to move forward.

Ben Comer profiles 10 innovative technologies designated as new and revolutionary tools for the treatment of disease and disability.

Given the amount of activity in the hepatitis C drug development space – as evidenced in PharmExec’s 2013 Pipeline report – patients are scouring the internet for information on the disease, available treatments, and soon to be available treatments.

How can the emerging field of data science help to fruitfully translate research and discovery into successful drug development.

Patients in search of face time with a healthcare provider are stopping into their local drugstores, where consultations are free and the wait time is shorter.

Is the 'lost decade' of declining pipeline productivity finally over? Our annual survey of analyst assessments of the state of drug development suggests so...
Ideology, politics, and a stilted political debate may be causing pharma to overlook the potential of emerging stem cell therapies in fostering a new generation of cures.

The most obvious competitors for Sanofi’s Aubagio, the second oral multiple sclerosis (MS) drug to receive FDA approval, would appear to be Novartis’ Gilenya.

“Doctor’s don’t hate technology, they hate bad technology,” said Shane Kennedy, managing director, digital, at Sudler & Hennessey.

The pharmaceutical industry still lags other industries in spending on outbound social media programs that engage customers directly.

Specialized knowledge about disease and treatment is no longer the exclusive province of practicing physicians. Patient voices are increasing in volume, along with their roles in treatment decisions.

It’s something of an enigma that most physicians are keen to learn about and use cutting edge medicines and devices for their patients.

In the American version of the Australian TV series Wilfred, the show’s title character declares that for him, human life begins at 10 years old.

Forest Labs has had a tough time with regulators over the last two years. The company’s sales reps can’t seem to stop breaking the law.

Can’t get your sales rep into that academic medical center? Try sending a medical science liaison (MSL).

At CBI’s 7th Annual Rare Disease and Orphan Drug Leadership Congress, speakers and attendees identified areas where pharma can improve the way it approaches rare disease and treatment.

Like governors in the US vowing to block Medicaid expansion in their home states, local primary care trusts (PCTs) in the UK don’t always follow national guidelines on drug access due to budgetary concerns.