
Lisa LeCointe-Cephas, SVP, chief ethics and compliance officer and office of general counsel, human health, Merck, uses her unique style of leadership—stop, drop, and roll—while elevating voices that need to be heard.
Lisa Henderson is the former group editorial director of Pharmaceutical Executive, Applied Clinical Trials, and Pharmaceutical Commerce.

Lisa LeCointe-Cephas, SVP, chief ethics and compliance officer and office of general counsel, human health, Merck, uses her unique style of leadership—stop, drop, and roll—while elevating voices that need to be heard.

In this exclusive Q&A with Pharmaceutical Executive, Janice Chang, CEO of TransCelerate BioPharma, provides an inside look into her career journey, her work at the company, how the industry has changed in the last 10 years, as well as her thoughts on where the industry should put its efforts in clinical trials.

Now with a seat at the table, growing accountability, and evolving competencies, medical affairs can use its far-reaching influence to change pharma for the better.

Workshop on pharma M&A review and enforcement focuses on what’s not working and what should be on the chopping block.

Complaints filed against CMS target the unconstitutionality of the Act, while the stifling of innovation in biopharma runs a parallel argument for numerous reasons

Ahead of ASCO and BIO, Michael Bailey CEO of AVEO Oncology, discusses the company becoming an LG Chem subsidiary and the future for drug development in the combined entity.
While ESG is still in the early stages for pharma, many companies are moving forward with a need for compliance.

Digital and innovation leader takes issue with the current state of pharma marketing and suggests new approaches are needed.

Industry insights on how pharma is faring in tapping new innovations and approaches in digital health.

Panel discusses what real-world evidence—and related patient-reporting technology—need to achieve to better influence payer decision-making.

Tool highlights efforts to overcome the challenges of remote disease management in Turkey.

Experts explore MA's evolving role and skill set in engaging effectively with clinicians and other external stakeholders.

German laws, European VBCs, and trends in China, Brazil, and Saudi Arabia are changing the way global market access works.

At Pharma USA 2023, Peter Marks, MD, PhD and director of CBER described FDA and public/private partnerships working toward scalable manufacturing approaches to address commercial viability and global access for cell and gene therapies

Co-Founder and CEO John Oyler discusses the business decisions BeiGene makes to grow its pipeline and address drug pricing and access.

Starting out with limited pharma business knowledge, how the lessons learned and realizations made helped to forge a destined—and globe-spanning—leadership path for Christophe Bourdon, where today, as CEO of LEO Pharma, he is tasked with steering the longtime company’s return to profitability.

Pharm Exec details the career of Rajesh K. Rajpal, MD, the chief medical officer and global head of clinical and medical affairs at J&J Vision.

Interpreting what the Inflation Reduction Act could mean for biopharma.

Experts in attendance at Pharma 2022 share insights on medical affairs.

Executives share insights at recent conferences.

In episode 119, Doina Ionescu, Managing Director, UK & ROI, at Merck, discusses her career journey, gender discrimination in the workplace, her role in the ABPI, and lessons she's taken from Queen Elizabeth II.

Top hubs continue to perform despite financial uncertainty in other industries.
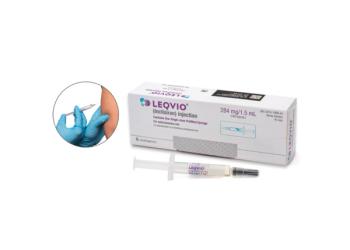
More favorable administration, reimbursement could separate latest PCSK9 entrant.

Dealing with discrimination and bias throughout her career, Otsuka Pharmaceutical’s Christine Sakdalan has forged a strong leadership path built around a two-pronged mission—finding true meaning in the patient journey through market research and data analytics, and mentoring and supporting the development of today’s aspiring professionals.

Motivated by forging new—and needed—pathways in drug development.

Physician-scientist turned Big Pharma medical chief sees fruits of labor.

Role drift and clinical trials among many areas for compliance professionals to keep an eye on in 2022.

Our latest CEO Leadership roundtable focused on the most top-of-mind issues for companies.

Pharma should take an integrated approach to representing cultural elements in materials.

Ted Schroeder, CEO of Nabriva Therapeutics, is passionate in his forward-thinking approach to finding the next generation of anti-infectives, as resistance to least-expensive, tried-and-true therapies continues to grow.

Published: January 11th 2023 | Updated:

Published: August 10th 2021 | Updated:

Published: June 9th 2021 | Updated:

Published: October 12th 2021 | Updated:

Published: November 9th 2021 | Updated:

Published: August 10th 2021 | Updated: