
Affiris struck a deal with GlaxoSmithKline to develop two Alzheimer's treatments. As part of the agreement, GSK gains a fancy protein technology that could be a boon for future molecules.

Affiris struck a deal with GlaxoSmithKline to develop two Alzheimer's treatments. As part of the agreement, GSK gains a fancy protein technology that could be a boon for future molecules.

IMS Special Report

New legislation gives US-based Web hosting companies the go-ahead to remove shady online pharmacies without a warrant. The new rule makes it harder to market counterfeit drugs, and for consumers to purchase medication without a prescription.

Novartis is the latest pharma company to move away from the traditional sales model of blanketing sales reps for one drug across the nation. The company is restructuring its force (and cutting jobs) to focus on a regional sales model.

Analysts report that Big Pharma isn't being hit that hard by the credit crunch due to the large amount of cash many companies have in their coffers for mergers and acquisitions. However, smaller firms looking to be sold may have a hard time holding out for higher offers.

Specialty drug firm Norgine splits from the British pharma association due to a growing rift about pricing. A "war of the roses" is escalating between Big Pharma and smaller drug manufacturers; Norgine could be the first casualty.

With the launch of Clinician 1, physician and nurse assistants now have a place to share information online. The increase in No-See doctors and general difficulty for reps to meet with prescribers might make this the opportunity for pharma to reach a major stakeholder.

The pharma giant just announced major plans to break up its R&D divisions into smaller units targeting areas of growth. Marketing will be part of the restructuring plan, and there is good news: No new layoffs.

Try this one on for size: Multi-tasking isn't the path to greatness

A head-to-head comparison of the Drug Vote '08

A vision of pharma's next business model is starting to emerge. But how do we get from here to there?

Cubist Pharmaceuticals CEO Michael Bonney has given the company a successful start-and is now predicting the future of drug resistance.

In an effort to give healthcare professionals and patients a one-stop source for factual drug safety information and treatment options, Pfizer has launched a handy new medicine safety Web site with a bevy of bells and whistles.

A new FDA Web site defines consumer print advertising so the general public can spot bad and good pharma ads. As a bonus, the site also serves as a checklist for pharma marketers trying not to step on any regulatory toes.

Glaxo's cancer departments will be consolidated into one big division encompassing discovery, research, and trials. The move is expected to cost some jobs, but GSK hopes it will strengthen synergy within its R&D unit.

A new study from the NIMH shows that next-generation treatments for schizophrenia might not be any better than older, first generation drugs-and in some cases might be worst.

A new study finds that doctors are three times more likely to prescribe generics when they don’t have access to free samples of branded drugs.

Celebrity spokesperson Dr. Jarvik has been replaced with a Regular Joe in the latest Lipitor DTC spots. Will the switch make a difference?

A new study shows that while the combo drug decreases the chance of coronary artery disease, it doesn't work as well in slowing down aortic valve disease. So why is all the focus on cancer deaths?

After a year of layoffs and restructuring, Valeant Pharma scores big with a collaboration deal worth up to $670 million.

The Belgium-based drug maker is terminating 2,000 jobs and rebranding itself as a biopharm with a focus on CNS and immunology drugs. Pharm Exec gave UCB a call to find out more.

Designed to rein in the relationship between sales reps and doctors, PhRMA's new, revised code may end up having an adverse effect on clinical trials

Effective January 1st, sales reps will have a new standard for dealing with doctors the PhRMA way

FDA missteps and global outsourcing are drawing Congressional scrutiny and prompting new oversight approaches
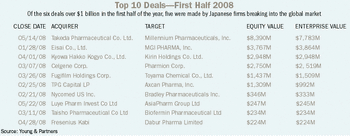
Young & Partners crunches pharma's filings for, the first half of '08. The results? Ask the Japanese

The Orphan Drug Act was passed 25 years ago. But the challenge of actually getting rare disease drugs and therapies to patients still remains

A new study tracks six high-traffic drugs that are turning up on shady online pharmacies, usually at huge discounts. The conclusion: Fancy Web sites are suckering customers and costing pharma money and credibility.

Tacked on to Genentech's press statement about its rejection of Roche's offer, the biotech firm announced the formation of a program to address employee concerns.

The case for preemption gets shot down in Texas, but holds up in yet another Medtronic suit in Minnesota.

Margaret McGlynn, Merck's head of vaccines, expounded yesterday on the state of the company's vaccine program, including campaign strategies, product development, and a new reimbursement program for doctors.