
Rather than wait to see which ePedigree legislation becomes law, biotech firm Genzyme has taken the initiative and implemented an electronic track-and-trace program to clamp down on fake meds.

Rather than wait to see which ePedigree legislation becomes law, biotech firm Genzyme has taken the initiative and implemented an electronic track-and-trace program to clamp down on fake meds.

The Hershey Center for Applied Research is bringing social networking to researchers and academics with a new platform that could become a Facebook for the life science community. Does the site, dubbed KnowledgeMesh, have what it takes cut through the Web 2.0 clutter? Pharm Exec takes a closer look.

Join the IMS workshop dedicated to a groundbreaking new approach to marketing.

Prix Galien Sponsorship

Marketers and sales reps feel the brunt of faltering Procrit sales as J&J consolidates its biologics divisions.

Consumer advertising might be taking a hit in the media and Congress, but pharma was all smiles at this year's PhAME Awards as the best DTC campaigns took the spotlight

Still hoping the journal article on your drug's adverse reactions will go unnoticed? Think again. A new partnership between medical journals and physician social networking site Sermo just made chatting about drugs a lot easier.
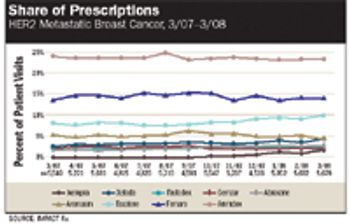
Tracking business by indication is critical for evaluating market performance and making accurate resource allocations
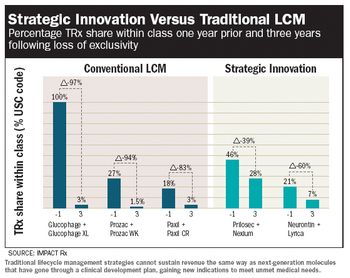
How to stretch that portfolio to make ends meet

FDA is being pressured to get its act together. The agency says it needs more money and more people to get approvals out the door
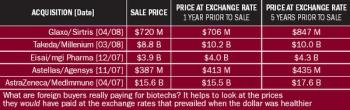
A plunging currency and other economic turmoil shouldn't phase pharma, right? Yes and no

Small biotech company is creating a buzz with a diabetes drug that incorporates a component found in red wine. Pharm Exec talks to Sirtris CEO Christoph Westphal about the merger and the battle against diseases of aging.

Bill Gates and Co. officially released Office for Business Applications (OBA) for the life sciences industry. This set of tools allows software developers to build applications that seamlessly connect back-end enterprise systems with MS Office programs.

As companies make headway with Web promotions, their marketers express concerns about how best to benchmark online efforts.

Blog entries about branded drugs-both positive and negative-are turning up in online keyword searches. Should pharma be worried?


Japanese drugmaker buys the cancer-specialist biotech for a whopping $8.8 billion.

GSK, Pfizer, and Merck have jumped on the cell-phone marketing bandwagon, but most of pharma is still tiptoeing around the technology. A new report details how pharma can get consumers to text for health info.

With M&As the new R&D and sales forces getting axed, the CFO is transitioning from background player to front man. A new survey documents the changing role of the pharma controller.

While some drugmakers are being pummeled for data on their "bad" cholesterol?lowering drugs, promising Phase II stats about its "good" cholesterol?raising CETP inhibitor has Roche smiling. Can it escape the torcetrapib curse?

California's plan for full-blown ePedigree implementation by 2009 just got a reality check. The hang-up? The systems are cost more and are taking longer than expected to get up and running.

Veteran salesperson Reggie Smith of Genentech makes the case for sales force diversity

Pharma is embracing the cross-functional team. But that doesn't mean that you must blend in with the sheep. Putting your best foot forward strengthens the team...and you.

International outsourcing carries risks for marketers and regulatory challenges for FDA

The real question in the Baxter heparin scandal is not who's guilty but whether pharma can get reliable results from a country in chaos

When it comes to developing novel therapies from the animal kingdom, it's mostly a game of pick your poison

The real question in the Baxter heparin scandal is not who's guilty but whether pharma can get reliable results from a country in chaos

A new study looks at the movement of thought leaders and medical science liaisons from primarily marketing to other areas of the pharma company. Though the shift might be minor (7 percent since 2002), it might be a sign of things to come.

Now that Apple has opened its iPhone to third-party software developers, healthcare productivity programs are leading the charge. Here?s a preview of one of the frontrunners.

What will the next president really mean for US healthcare policy? PwC's Sandy Lutz reports.