
All News





The importance of early development strategy and planning for biologic patent term extension.

Click the title above to open the Pharmaceutical Executive July 2018 issue in an interactive PDF format.


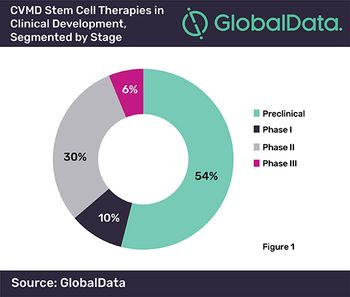
Reputation Institute study finds that the reputation of pharma companies is in decline and might get worse before it gets better.

My first gathering of Pharm Exec's Editorial Advisory Board was both an honor and a learning experience-about us, the industry, and maintaining our mission to the our readers.



Click the title above to open the Pharmaceutical Executive June 2018 issue in an interactive PDF format.


In this demanding environment, yesterday’s product launch playbook can no longer be relied upon to yield the expected patient or business outcomes, writes Boris Bogdan.

A discussion of the benefits that a cloud-based strategy has to offer.

This article is a roundtable consisting of global Medical Affairs leaders to discuss the potential of Medical Affairs as a key value driver and the role MAPS will play in bringing that potential to fruition.




Ellen Coleman outlines the highlights of a recent webinar on ensuring that patient advocacy is a key element of a market access strategy.


A variety of consultant options are available to the pharma industry; this article is a 3-step guide to selecting the right consultant.



Click the title above to open the Pharmaceutical Executive May 2018 issue in an interactive PDF format.


Insights from Partnering with IDNs: Biopharma Strategy Summit.
