
All News


Click the title above to open the Pharmaceutical Executive August 2017 issue in an interactive PDF format.







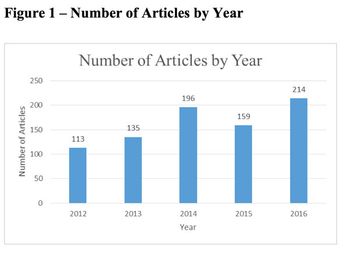
If it feels like the media’s scrutiny of pharma has intensified recently, there's a reason-it has, as the results of our 13th Annual Press Audit indicate.

As digital technologies transform drug development and marketing, a retooled medical affairs function can be a competitive differentiator.
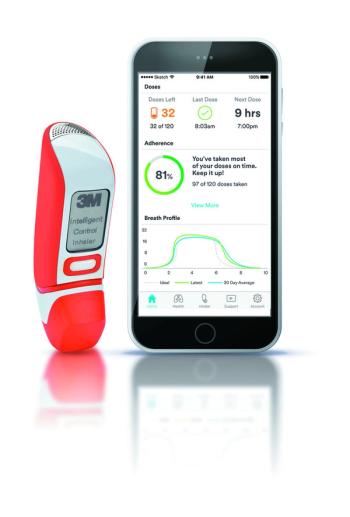
Highlighting a sampling of new digital health devices that span the wide focus terrain in health technology.

Click the title above to open the Pharmaceutical Executive July 2017 issue in an interactive PDF format.









These articles from Pharmaceutical Executive help will help marketers stay on message and, crucially, up to date in a field that is changing faster than ever.







