
HTAs are gridlocked by diverse national requirements, a lack of alignment on processes, and even on the value of their outcomes.

HTAs are gridlocked by diverse national requirements, a lack of alignment on processes, and even on the value of their outcomes.

What India's latest census tells us about current and future pharma opportunities.

While Europe's EFPIA preaches about embracing change, much remains to be done to prove the adjustment in philosophy

A country that bridges East and West, old and new-while boasting a growing economy in Europe-is being looked to for its pool of talented industry resources

Survey on European market access trends says the most challenging factor in the pharma industry is switching focus on marketing to the payer rather than the prescriber.

GlaxoSmithKline has entered into an agreement to purchase Shenzhen Neptunus? stake in a previously formed joint venture between the companies involved in the development and manufacture of influenza vaccines in China, Hong Kong, and Macau.

Is it a coincidence that the ABPI has netted a whole shoal of conduct contraveners in the first month its amended Code of Practice became operational?

Why has the EU still not managed to update its rules on information about medicines?

Hungary?s failure to advance EU legislation on information to patients is not the country?s fault, says Reflector.

Rising income per capita and a budding consumer health consciousness are fueling a CAGR of 9.5%, giving investors plenty of room to expand market penetration

The UK coalition government's consultation on the intro of value-based pricing has invited caution and resistance

The EU is largely powerless when it comes to policies over pricing and reimbursement
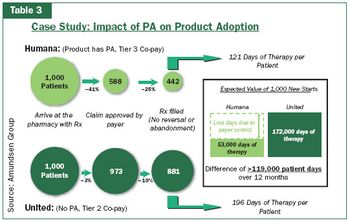
The pharmaceutical industry needs to use new and better data to accurately measure how much it is willing to invest in avoiding plan control

In France, it took a president's keynote speech to underscore the government's new understanding that there is more to pharmaceuticals than cost-containment

Philippa Montgomerie outlines the implications of the Court of Justice of the European Union's recent patent litigation forum shopping.
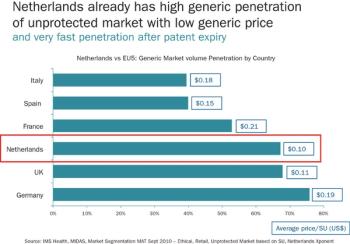
Despite its dimunitive size, the pharmaceutical industry looks to the Netherlands for a world-class medical infrastructure, new standards in innovation, and a closely-knit community of stakeholders

Pharma can be an insular industry, with limited capacity to gaze beyond its own narrow frame of vision

Head of the Publications and Multimedia Department

To manage through a resource-constrained environment, it is essential to get the most out of procurement planning

January 10, 2011.

The mediterranean nation has endeared itself to foreign businesses over the past decades, adtively positioning itself as an appealing epicenter of global pharma interest
The Oxford Finance Programme for Senior Executives is for directors from technical backgrounds involved in strategic planning, and delivers the ability to identify which projects to invest in, retain or divest.

After many years of building up an impressive resource base, the industry is finally ready to take advantage of the wave of enthusiasm and interest surounding pharma and biotech that is sweeping the nation

A small company shows how it can play a major role in a relatively short time frame

Outgoing EMA Director Thomas Lonngren discusses a decade at the helm of Europe's top regulatory office