
Pharma companies have opportunities to grow, but they also must plan for a complicated future.

Pharma companies have opportunities to grow, but they also must plan for a complicated future.
The Refusal to File letter comes despite the FDA’s prior encouragement to submit a supplemental biologics license application for Anktiva in patients with BCG-unresponsive non-muscle invasive bladder cancer with papillary disease.

In the face of potential tariffs, the pharmaceutical industry must find alternative ways to reduce costs.
Selarsdi, a biosimilar to Stelara, is approved for adult and pediatric psoriatic arthritis, plaque psoriasis, Crohn disease, and ulcerative colitis.

The investigation would determine whether excessive imports pose a threat to national security.
AbbVie secures FDA approval for Rinvoq as the first oral Janus kinase inhibitor indicated for giant cell arteritis, expanding its immunology portfolio and signaling strategic growth opportunities in underserved autoimmune markets.

IQVIA’s Doug Long offers a lay of the land, including trends and developing issues that are shaping the current market.
Approval of penpulimab-kcqx marks the company’s US regulatory debut and introduces a new immunotherapy option for advanced nasopharyngeal carcinoma.

The chaotic tariff situation is forcing pharma companies to prepare for multiple outcomes.
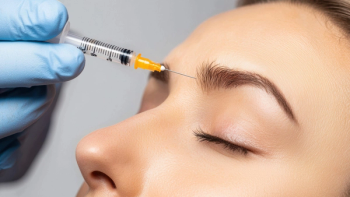
The submission is backed by clinical data from over 2,100 patients, including two pivotal Phase III trials in patients treated with trenibotulinumtoxinE for moderate to severe glabellar lines.

The simplified requirements for Camzyos are expected to ease prior contraindications, thereby simplifying treatment and expanding eligibility in patients with symptomatic New York Heart Association Class II-III obstructive hypertrophic cardiomyopathy.

Dupixent is the first new targeted therapy to receive FDA-approval for chronic spontaneous urticaria in adults and adolescents over 12 years of age in over a decade.

Regulatory action was based on data from the Phase III QUASAR trial, which demonstrated that Eylea HD dosed every eight weeks achieved non-inferior visual acuity outcomes compared to Eylea in patients with macular edema following retinal vein occlusion.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to expand access to Pfizer’s respiratory syncytial virus vaccine Abrysvo for high-risk adults in their 50s and voted in favor of GSK’s meningococcal vaccine, Penmenvy, for streamlined adolescent protection.

Four tips for midsized companies when entering new global markets.

Sonam Dubey, a partner at Beghou Consulting, discusses the potential impact of a possible ban on direct-to-consumer advertising amid policy and government agency overhauls in the US.
Approval of Opdivo plus Yervoy combination was based on results from the Phase III CheckMate-9DW trial, which demonstrated significantly improved overall survival and overall response rate in patients with unresectable or metastatic hepatocellular carcinoma.
Full approval of Vitrakvi was based on results from three clinical trials in patients with unresectable or metastatic NTRK fusion-positive solid tumors.

As the physical products we use evolve to become increasingly complex, traditional products liability frameworks may not always fit to provide remedies for harm that can result from using novel product types.
Approval of the Opdivo plus Yervoy combination regimen was based on results from the Phase III CheckMate-8HW trial, which was the largest immunotherapy study in patients with previously untreated, unresectable, or metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer.

Brimochol PF, a combination of brimonidine and carbachol, was found to enhance depth of focus and visual acuity in clinical trials among patients with presbyopia.

The survey also suggests that Medicaid is broadly popular amongst all Americans, regardless of political affiliation.
Results from Phase II studies demonstrated that rilzabrutinib showed clinically meaningful outcomes in patients with warm autoimmune hemolytic anemia and IgG4-related disease.

Results from the Phase III SPACE trial show that Ajovy significantly reduced monthly migraines in pediatric patients between six and 17 years of age.

Eli Lilly CEO David Ricks to the BBC: “It feels like it'll be hard to come back from here."