
In this Pharmaceutical Executive video interview, Jen Butler, Chief Commercial Officer for Pleio, shares some best practices on finding trustworthy and verified information about a new prescription or medical device.

In this Pharmaceutical Executive video interview, Jen Butler, Chief Commercial Officer for Pleio, shares some best practices on finding trustworthy and verified information about a new prescription or medical device.

The application leverages existing clinical data for Uzedy along with prior FDA findings on the safety of Udezy in patients with bipolar I disorder.

In this Pharmaceutical Executive video interview, Jen Butler, Chief Commercial Officer of Pleio, discusses the significant threat of misinformation to public health and how emotional appeal plays a part.

Surgifort is the first FDA-approved human milk-based fortifier specifically designed for term infants recovering from gastroschisis surgery.
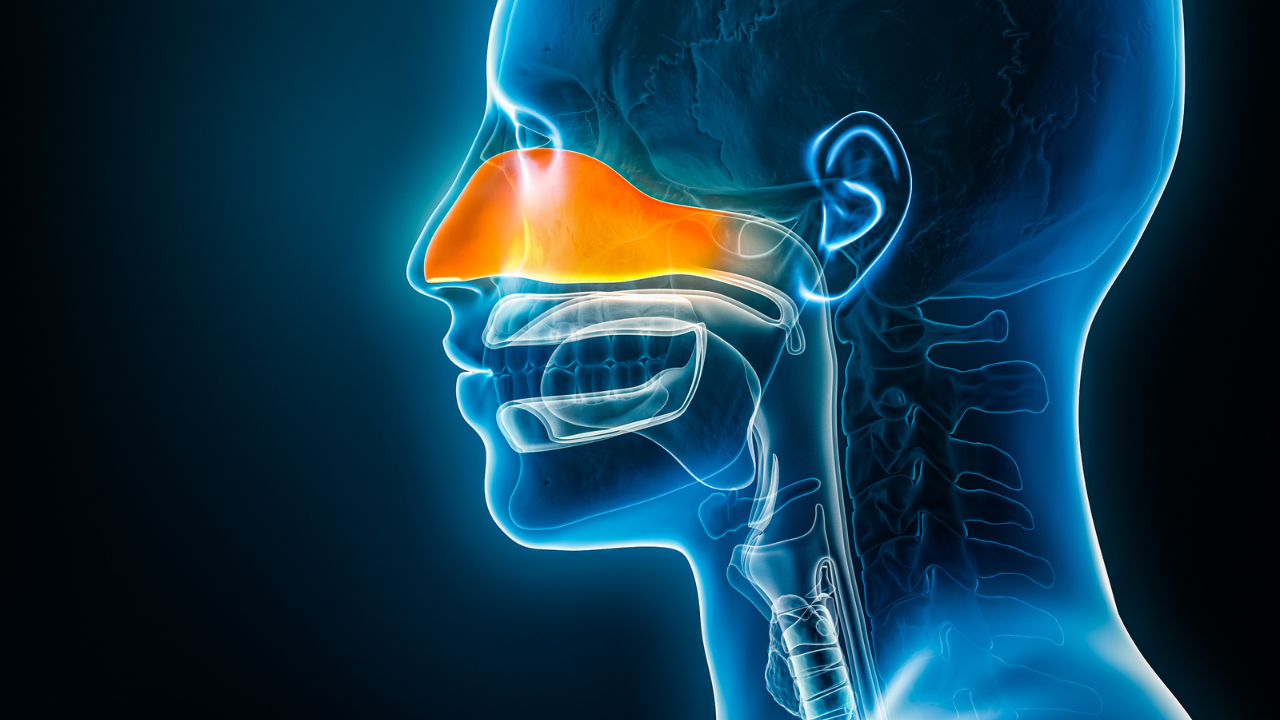
Keytruda demonstrated a statistically significant and clinically meaningful improvement in event-free survival and major pathological response in patients with resectable locally advanced head and neck squamous cell carcinoma.

The BrainSense adaptive deep brain stimulation system personalizes therapy by dynamically adjusting stimulation based on real-time brain activity to improve Parkinson disease symptom control without manual adjustments.

Launching this month, Vaxitek HVT+IBD+H5 integrates COBRA technology to address the ongoing viral evolution challenges of avian influenza, as well as for the prevention of Marek disease and infectious bursal disease.

Biopharmas and global agencies are now prioritizing initiatives and innovations to drive speed across product development with an emphasis on getting new treatments in the hands of doctors and patients.

In this Q&A, Organon's Chief Communications Officer, Becky Edwards discusses how much social media and influencers sway decisions about birth control methods, potential consequences of these misinformed choices, and more.

Former leaders of the CDC, FDA, and other agencies condemned the layoffs.

James Foster, CEO and co-founder of Virax Biolabs, discusses how global launches are being impacted by changing geopolitical conditions.
Ospomyv, a biosimilar to Prolia, is indicated for postmenopausal women and men at high risk of fracture, while Xbryk, a biosimilar to Xgeva, is indicated for preventing skeletal-related events in multiple myeloma and solid tumor bone metastases.

Vimkunya is the first FDA-approved vaccine for chikungunya in patients over 12 years of age.

Ian Baer, Founder and CEO of Sooth, discusses how the growing distrust in social media will impact industry marketing strategies and the relationships between pharmaceutical companies and the patients they aim to serve. He also explains dark social, how to combat misinformation, closing the trust gap, and more.

John Arena, interim president of global pharma services at Cencora, discusses how companies can continue to develop launch strategies while taking potential regulatory changes into consideration.
Meilog marks the first rapid-acting insulin biosimilar to gain FDA approval for the treatment of diabetes.

The latest news for pharma industry insiders.

James Foster, CEO and co-founder of Virax Biolabs, discusses how companies can strategize despite hard-to-predict regulatory headwinds.
Approval of Adcetris provides a new treatment option for patients with relapsed or refractory large b-cell lymphoma who are ineligible for autologous stem cell transplantation.

James Foster, CEO and co-founder of Virax Biolabs, discusses how the industry is preparing for potential regulatory changes to vaccine policy.
Evrysdi is a non-invasive, disease-modifying option that can be swallowed whole or dispersed in water for patients with spinal muscular atrophy.

Pathways to success for this key component of Europe’s new HTA regulation, where thinking beyond the purely technical will be critical.

In this part of his Pharmaceutical Executive video interview, Ian Baer, Founder & CEO of Sooth, identifies strategies pharmaceutical companies can use to combat misinformation on social media.

Approval makes Gomekli the first treatment to be approved for both adults and pediatric patients for neurofibromatosis type 1, a genetic disorder that causes noncancerous tumors to grow on nerves throughout the body.

A change in political power is altering how companies are strategizing around the IRA.