The full approval of Spikevax covers children aged six months through 11 years who are at increased risk for severe COVID-19, transitioning the vaccine from emergency use to full licensure.


The full approval of Spikevax covers children aged six months through 11 years who are at increased risk for severe COVID-19, transitioning the vaccine from emergency use to full licensure.
Approval was based on results from the DOORwaY90 trial, which demonstrated a 98.5% overall response rate and 100% local tumor control in patients treated with the SIR-Spheres Y-90 resin microspheres for unresectable hepatocellular carcinoma.

Justin Kozak discusses how the impact on medical breakthroughs and startups.
Ekterly becomes the first FDA-approved oral on-demand treatment for hereditary angioedema in patients aged 12 years and older.

The organization says that millions of Americans will lose access to mental health care.
Accelerated approval was based on results from the Phase I/II LINKER-MM1 trial, which showed a 70% objective response rate in patients with relapsed or refractory multiple myeloma treated with Lynozyfic.
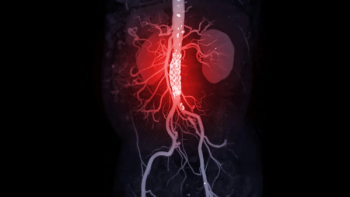
Results from the Phase III ZENITH trial show that patients treated with Winrevair (sotatercept-csrk) for pulmonary arterial hypertension experienced a 76% reduction in the composite risk of death, lung transplant, and ≥24-hour hospitalization.
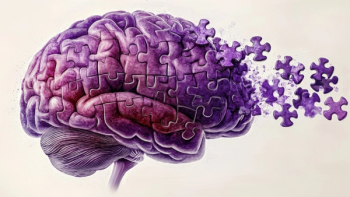
Updated label allows Neuraceq to be used in selecting patients for amyloid-targeting therapies and includes the use of quantitative PET imaging metrics to support diagnosis and monitor disease progression in Alzheimer’s care.
Jeff Liter, CEO of Luminary, discusses his company’s approach to CAR-T therapies and why the developing Saudi market has potential.
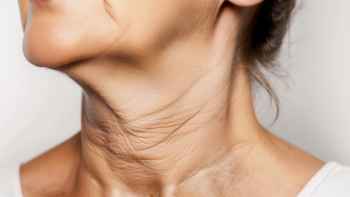
The supplemental premarket approval application is supported by Phase III data, which showed significant improvements in neck appearance in patients treated with Skinvive.
Gamifant marks the first-ever FDA-approved treatment for both adult and pediatric patients with hemophagocytic lymphohistiocytosis macrophage activation syndrome in the context of known or suspected Still's disease.

New rules places upon the pharma industry will have a significant impact on how investors view the industry.
The recommendation for Enflonsia is indicated for respiratory syncytial virus prevention in infants under eight months of age, expanding protection options ahead of the 2025–2026 season.
Label changes remove Risk Evaluation and Mitigation Strategy programs and eases monitoring requirements, supporting broader access to Bristol Myers Squibb’s CAR T-cell therapies Breyanzi and Abecma for patients with large B cell lymphoma and multiple myeloma.

Q&A with Model N's Michael Grosberg drills deeper into the new US drug pricing directives, including challenges in implementation, the potential unintended consequences, and how these actions could spark the shift to outcomes-based contracting in healthcare.

The label update for Amyvid adds guidance for assessing amyloid plaque levels and may help inform diagnostic and treatment decisions in patients being evaluated for Alzheimer disease.

The idea of denying people proven medicines in the name of research has a dark legacy in this country.
Approval was based on results from the Phase II TROPION-Lung05 trial, which showed that Datroway demonstrated a strong overall response rate and duration of response in patients with previously treated, locally advanced or metastatic EGFR-mutated non-small cell lung cancer.
Approval was based on results from the Phase III inMIND trial, which demonstrated a statistically significant and clinically meaningful improvement in progression-free survival in patients treated with Monjuvi combined with rituximab and lenalidomide for relapsed or refractory follicular lymphoma.

Dupixent is the first and only targeted therapy to receive FDA approval for bullous pemphigoid.

Harliku becomes the first FDA-approved treatment for alkaptonuria, indicated to reduce homogentisic acid levels in affected adults.
Approval of first twice-yearly HIV pre-exposure prophylaxis was based on results from the Phase III PURPOSE 1 and PURPOSE 2 trials, which show that ≥99.9% of patients treated with Yeztugo remained HIV-negative.

The investigational MRI contrast agent, gadoquatrane, is designed to deliver effective imaging at a significantly reduced gadolinium dose, supporting safer repeat use in adults and pediatric patients.

Special Guest Opinion: A reimagined regulatory framework for the life sciences is not just pro-investor—it is pro-patient.

Under the new initiative, companies may receive a voucher enabling FDA review to be shortened from the standard 10–12 months to just 1–2 months following final application submission if the drug addresses US national health priorities.