
Taking stock of evolving practices in protecting the supply chain amid the pandemic, including partnering and planning for what’s next.

Taking stock of evolving practices in protecting the supply chain amid the pandemic, including partnering and planning for what’s next.
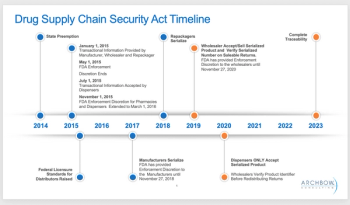
It is vital to begin asking questions about your company’s DSCSA readiness and compliance, as well as that of your strategic partners, writes Rob Besse.

MilliporeSigma's Robert McGuirk speaks with Pharma Exec about the role of supply chain robustness in helping to mitigate the risk of drug shortages

Coexisting with the DSCSA may hinder new legislative efforts.

How to navigate the production and reimbursement intricacies of bringing regenerative medicines from bench to bedside.

Tim Willis and Oliver Wack outline the role of alternative data in supply chain risk management, and the ancillary benefits that come from adopting such technology.

The next generation of supply chain shows an increasing need for blockchain technology.

How low-cost drugs can succeed in the specialty pharmacy channel.

In today’s drug development environment, there is no doubt that supply chain decisions are paramount in a product launch strategy, writes Lisa Henderson.

Steve Cottrell assesses the risks of lapses in pharma's supply chain visibility and outlines what can be done to minimize them



How a Manufacturing Excellence program can deliver competitive advantage to a fast-changing global product mix-at lower cost, and with an unexpected workforce dividend to boot.

Pharm Exec sits down with AmerisourceBergen supply chain leader Peyton Howell to discuss the latest trends in pharmaceutical distribution, including company efforts to boost efficiency, reliability, and enhance its groundbreaking partnership with Walgreens Boots Alliance.

Limitations of the Drug Supply Chain Security Act for pharma-and three strategies to fix them.

The recent formation of three mega supply-chain purchasing groups (Walgreen’s-AllianceBoots-AmerisourceBergen-Rite-Aid, McKesson-Celesio, CVS-Cardinal) denotes a shift in the balance of power between wholesalers, chains, generic pharmaceutical companies and branded pharma companies.

An advocate of business system automation in the global pharma supply chain offers a six-pronged approach to adapting to the realities of a new age of mounting regulation and customer expectations.

A Pharm Exec conversation with AmerisourceBergen CEO Steve Collis

Leaders should understand the key themes of risk intelligence in the supply chain, writes Bronwyn Kunhardt.

Kathleen Iacocca and Yao Zhao discuss why direct supply chain models can be more sustainable than resell models in the long run and whether they can be implemented in the US.

New legislation and changes in policy at FDA are leading to better control of the API supply chain.

Examining a new three-part formula to enable the supply chain function to manage market pressures while delivering on the bottom line.

As insurers, payers, and drug plan operators eye increased generic drug use as key to cutting healthcare costs, pharma companies are ramping up efforts to delay marketing of copycat products.

Customer-focused supply-chain capabilities are becoming a more important part of a company's competitive advantage.

After years of asking questions, the National Highway Traffic Safety Administration (NHTSA)is moving closer to a protocol "for assessing the driving-impairment risks of drugs". Ben Comer reports.