
Finding the R&D sweet spot.
Casey McDonald is Senior Editor, Pharmaceutical Executive.

Finding the R&D sweet spot.

Masters of the deal converge in New York to discuss the status of their trade and the forces propelling M&A, licensing, and partnerships in the life sciences for 2015.

May 31, 2015.

A hot commodity, the FDA’s Rare Pediatric Disease Priority Review Voucher program once again proved its worth, with today’s $245m transaction.

Casey McDonald reports on yesterday's Healthcare Businesswoman’s AssociationWoman of the Year award ceremony in New York

Casy McDonald looks forward to CBI's Patient Adherence and Access Summit in Philadelphia.

Neuroscience luminaries clamored onto the stage at this week's World Medical Innovation Forum in Boston to present the meeting’s much-anticipated "Disruptive Dozen".

Pricing and Sovaldi couldn’t avoid mention at this week’s World Medical Innovation Forum put on by Partners Healthcare in Boston. The meeting highlighted multiple issues in neuroscience but also touched on hot buttons across the industry.

Is it time to view cell therapy companies differently on Wall Street-even distinct from biotech?

Although the strategic hurdles of biosimilars market entry are not new, the task of imitating substantially more complex molecular entities offers up a host of new operational questions for pharma.

March 12, 2015.

In reaction to AbbVie’s massive acquisition of Pharmacyclics, investors who were caught unprepared are looking for ways they could have predicted the deal. Casey McDonald looks at the reaction on Twitter.

The cholesterol-fighting market is back with a vengeance; plenty of complications in store

The interesting dynamic of industry-targeted memes in social media-still a quiet treading ground for pharmas.

Fresh off Shire’s announcement to purchase NPS, CEO Dr. Flemming Ornskov sat down with PharmExec in San Francisco during the JP Morgan Healthcare Conference.

As we wrap up the week in San Francisco, here’s PharmExec's brief rundown of some of the top tweets of the week from JP Morgan and Biotech Showcase.

Pharm Exec’s annual report highlights the key scientific, commercial, and reputational trends shaping the industry in the year ahead.

Not all companies are big, small companies are scared, and myths drive behavior, stated Kay Holcombe, Senior Vice President, Science Policy at Biotechnology Industry Organization (BIO).
Cleveland Clinic's Pick of Top 10 Advances for 2015.
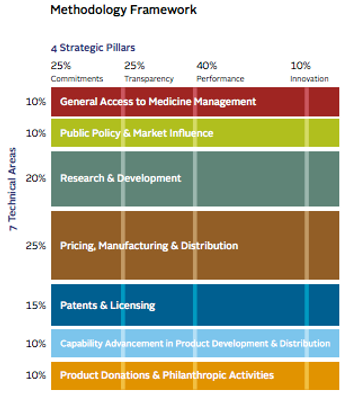
Last week, the Access to Medicine Foundation released its 2014 Index rating the performance of companies in broadening access to medicines in low and middle-income countries.

CytRx announced on Tuesday that FDA gave notice of a partial clinical hold for trials for its oncology candidate aldoxorubicin.

A cool $2.6 billion is the going rate to develop and gain market approval for a drug, according to the recent study by the Tufts Center for the Study of Drug Development (CSDD).

Merck KGaA CEO Stefan Oschmann spoke last week in New York as he accepted the two-year role as president of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), the R&D industry’s global trade group based in Geneva.

The Cleveland Clinic once again ended its Medical Innovation Summit with a list of 10 advances voted most likely to have a major impact on improving patient care in 2015.

Last week’s Prix Galien Forum on global issues in life sciences was not without controversy.

An illustrious panel of investment professionals spoke optimistically on the orphan disease space in spite of concerns surrounding escalating drug prices in specialty pharmaceuticals.