
RFID is not ready for prime time anywhere. Certainly not in the US. There is no way RFID gives you end-to-end control of the product.

RFID is not ready for prime time anywhere. Certainly not in the US. There is no way RFID gives you end-to-end control of the product.

California has proposed legislation for a pilot program to reinforce access to treatment for mentally ill offenders. This is a step in the right direction, but it should be the subject of national policy, not a localized effort.

Increased demand for plant-based hormones has spurred Wyeth to ask FDA to set marketing limits on compounded therapies

Home and abroad, the US Pharmacopeia is stepping up to maintain quality control. But it's not so easy. USP's Roger Williams discusses Medicare formularies, drug safety, international drug production, and the organization's changing role.
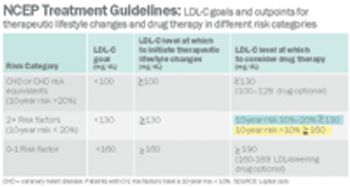
The world's largest drug manufacturer must answer off-label promotion charges brought by a new adversary. Not FDA, with its warning letters and threats of marketing sanctions, and not the Office of the Inspector General (OIG) at Health and Human Services, which often sues for fraud, forces huge settlements, and requires companies to do business under restrictive corporate integrity agreements. Instead, the company faces a class-action civil suit from insurance companies and union welfare funds, groups that, until recently, Pfizer regarded primarily as customers-or at least people who picked up the tab for customers. Now, led by the Welfare Fund of a Teamsters local from New Jersey, third-party payers are suing under RICO, the Racketeering Influenced and Corrupt Organizations Act. If their suit is successful, payers who have covered billions of dollars worth of Lipitor (atorvastatin) over the past five years will receive treble damages for the cost of off-label prescriptions. The suit may also attract..

Criminal penalties for violating the Foreign Corrupt Practices Act can be substantial. Businesses found guilty may be fined upwards of $2.5 million for each offense, or twice the amount gained as a result of the violation.

The pharmaceutical companies in the Predictive Safety Consortium have agreed to cross-test each other's laboratory methods to determine which are most effective in detecting kidney, muscle, and liver toxicity.

Where is pharma going? Toward more science, and more political pressure on science. Toward greater patient responsibility-and more regulation-by-lawsuit. And forward. Let's not forget about forward.

One health insurance company in The Netherlands is offering doctors a financial incentive to prescribe generic statins and proton pump inhibitors. Doctors and patients complained, but a court upheld the practice.

FDA doesn't usually write a black box warning "without some pretty good data," said Robert Temple of FDA's Office of Medical Policy. Overuse of the black box can both dilute its impact and limit access to needed treatment.

Drugs are not directly addressed in Europe's new legislation for chemicals. But they'll be affected, because chemicals are used in drug manufacturing.

Pharmaceutical companies are worried that FDA's new labeling format will expose them to liability suits from individuals who suffer adverse events that are not highlighted, but "hidden" in the full prescribing information.

While CMS officials praise the CED policy as a way to extend Medicare coverage, patients and industry fear the agency won't reimburse for innovative treatments unless sponsors support post-approval studies.

Is the public ready for an exciting thriller about terrorism, drug counterfeiting, valiant FDA agents, and glamorous women who invite pharma CEOs to come up and look at their drug patents? Sure. Maybe someone should write one.

The omission of criminal charges for off-label promotion of Serostim is surprising, because the government's earlier plea with Pfizer sent a strong signal that it would criminally charge companies engaged in off-label marketing.

Often, post-approval marketing studies don't materialize because drug companies question their value. Independent review of the need for such studies would address pharma's concern that they may be warranted.

To Janet Woodcock, Critical Path hasn't been dying, it's been getting work done."People shouldn't underestimate the difficulties of creating new models. A lot of things worth doing can't happen quickly."

FDA wants flexibility to use user-fee revenues to extend drug safety oversight, to review direct-to-consumer advertising before it goes public, and to modernize the ever longer and more complex drug development process.

Serious offenders of the new Association of the British Pharmaceutical Industry's Code of Practice can be "named and shamed" with adverts in the trade press. Details of breaches by companies will be posted online.

Patient-Program Interface Nearly one-third (2.5 million) of the estimated eight million Americans currently enrolled in PAPs are over age 65 and will be eligible to participate in the government's Part D program.

The public is focused on drug safety, but the real issue isn't approval standards. It's the way drugs expand to unapproved new indications. Are you listening, Acomplia?

FDA may be receiving fewer new drug applications for truly innovative products, but it has been overwhelmed this year with 800 abbreviated NDAs for generic drugs and thousands of supplements.

Despite heightened scrutiny from industry advocates and the beginnings of self-imposed regulation, pharma companies' violations of DTC regulations have been getting worse, says Tom Abrams, director of FDA's Division of Drug Marketing, Advertising, and Communications (DDMAC). Abrams has been on all sides of drug marketing, from receiving promotions as a pharmacist to creating promotions as a member of industry to regulating promotions as the head of DDMAC. As such, he's in good position to see the big picture.

The circumstances of Crawford's departure may complicate the process of securing a permanent FDA leader. Congress and HHS are investigating whether his confirmation process failed to uncover important facts.

Genentech and Biogen's MabThera has been awaiting NICE appraisal for three years. Merck KGaA's Erbitux has waited two-and-a-half years. A ruling on AstraZeneca's Arimidex isn't expected for more than a year.