
FDA has approved the medication in adults and children with allergies.
Mike Hollan is an assistant managing editor for Pharmaceutical Executive and Medical Device and Technology and can be reached at mhollan@mjhlifesciences.com.

FDA has approved the medication in adults and children with allergies.

The site will have a staff of 400 employees.
The agency issued a statement reminding the public that it has not approved any such devices for this use.

The gallery will be a part of the HLTH Europe event and will be open to all attendees.

The Gilead Sciences CEO will be joined by new board members from Pfizer and Sanofi.

Kaufman discusses the ways the digital biomarkers are improving Alzheimer’s research by directly tackling some of the unique challenges that researchers face.

Papa will take over the over effective immediately.

The previous CEO, Richard A. Gonzalez, has served as CEO since 2013.

Anderson discusses the complexities of planning for a global launch and the common mistakes companies make that cause problems along the way.

Wong discusses trends regarding M&A and how he expects this to continue and change in the coming months.

Lightburn discusses his company’s work with psychedelics and the unique business strategies required for these treatments.
Iris Kuss, head of clinical development, and Jorge Ortiz, head of medical affairs, discuss the company’s recent presentation at ASCO GU.

This acquisition will add asthma treatment A1O-001 to GSK’s portfolio.

Its path and application could model the same road as AI.

Harpreet Gill, vice president of real-world solutions - project management at ICON, discussesthe progress and challenges in advancing data-driven tools and approaches in decentralized clinical trials, where ongoing education remains paramount.
Bruce Phelan of Blue Fin discusses pharma’s need for access to manufacturing sites.

A new survey of pharma executives suggests that many are fully embracing the use of AI and are making financials plans based on the technology’s usage.
BioNTech will invest $200 million and get access to Autolus’ manufacturing sites.

According to the study conducted by Technavio, North America will contribute to 37% of the expected growth.

The three-year initiative comes with $15 million in funding.

The agreement will strengthen Novartis’ oncology pipeline.
The report predicts a 77% increase in global cancer cases by 2050.

The drugmaker will directly acquire three finish-fill sites as part of the deal.

The agency issued the warning against three brands of unapproved eyedrops designed to look like an approved brand.
The company has struggled to supply the drug to patients due to overwhelming popularity.

The shake is designed to work with regular dieters and those taking weight loss medications.

Researchers noted the onset of symptoms in patients who were treated with contaminated HGH therapy.
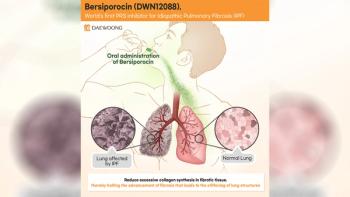
The agency recognized the medication as a significant advancement for the treatment of IPF.

Dr. Russo discusses how modern innovations will shape the way hospitals operate in years to come.

Sasser discusses how AI is directly helping with problems specific to precision and personalized medicine research.