
The acquisition will bolster AstraZeneca’s ability to develop radioconjugates for cancer treatments.
Mike Hollan is an assistant managing editor for Pharmaceutical Executive and Medical Device and Technology and can be reached at mhollan@mjhlifesciences.com.

The acquisition will bolster AstraZeneca’s ability to develop radioconjugates for cancer treatments.
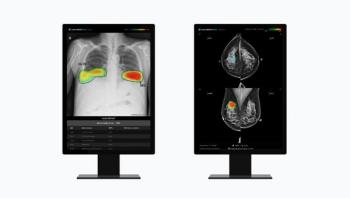
The company’s software will be used to help with chest and breast cancer screenings.
The technology was used to develop new peptides.
The therapies are still in the early stages of development, but researchers are optimistic about the results.

As part of the partnership, BeiGene will serve as a sponsor at LLS events.

The online retailer will be able to provide customers with a selection of migraine, obesity, and diabetes medications.

The study, out of Sweden, researched the impact of the use of medication and mortality rates for patients with ADHD.

The platform will allow for a more direct method of antibody optimization.

Cosgrove discusses how a recent study utilized a high-tech data platform to bring a new perspective on data to the researchers.

The voluntary recall was ordered due to potential silicon particulate exposure.

Sapirstein discusses the current conditions that are impacting investors’ view of the life sciences industry.

Classic views on leadership are sometimes still the most effective.

Ricciardi discusses how new treatments require much more specific patient groups in studies.

Tucker discusses the possibilities that non-hallucinogenic psychedelics bring to the sector.
The medical side of the market is growing while the commercial side seems to be struggling.

Anderson discusses how AI can be used to take data collected by different processes and bring it into a usable format.

The winner will receive $1 million and the chance to partner with a major industry leader.

Researchers are debating whether to still include protection for this strain in vaccines.

Chases discusses how traditional media platforms still coexist with digital platforms and how this impacts promotion.

The company plans to spend the coming years strengthening its market position and pipeline while also clearing its issues with litigation and debt.

Heavey discusses how the industry can avoid having efforts to accelerate discovery and development be hampered by manufacturing concerns.

Rus discusses his company’s work with neuroplastogens and how they differ from similar, psychedelic treatments.

The partnership has the goal of improving patient education and treatment directions.

The paper discusses multiple steps the industry can take to ensure that patients have access to these treatments.

Stark discusses how the ways that data is reshaping commercial strategies.

Simon discusses a recent survey ICON conducted with professionals in obesity-related clinical research.
The agency updated its recommendations after a vote by the ACIP advisory committee.

The agency teamed up with the software company to develop platforms and networks to collect de-identified data.

The device can detect potential warning signs of serious conditions.

The medication already has a black box warning on it, but the AG’s office believes stricter actions should be taken.