
The pharmacy will now operate as Saveway Compounding Pharmacy, a Myonex company.
Mike Hollan is an assistant managing editor for Pharmaceutical Executive and Medical Device and Technology and can be reached at mhollan@mjhlifesciences.com.

The pharmacy will now operate as Saveway Compounding Pharmacy, a Myonex company.

These expansions include a new product, along with improvements to existing products.

The move is intended to improve access to care at these critical sites.
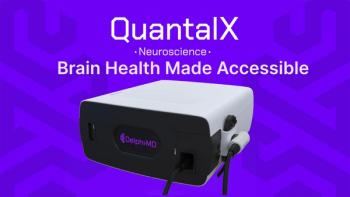
FDA’s program will provide guidance to the development of the company’s Delphi-MD technology.

The technology will reportedly improve consumer engagement.
The vaccine can reportedly prevent infection from a fungal source that impacts both people and dogs.

The two programs will be offered to students and CVS interns.

Otsuka and Click Therapeutics co-developed Rejoyn, a digital therapeutic for MDD.

Erickson discusses his work developing unique, targeted autologous and allogeneic T cell therapeutics.

The two company’s will provide consumers with a platform where they can compare drug prices.

Mills is working in leadership in two roles, one of which puts her on the forefront of the genomic medicine movement.

The two organizations will work together to advocate for better health outcomes for children and families in North Carolina.

The network will work with DiRx and MakoRX.

The news comes less than a year after Pfizer announced that IPG would handle this role.

The study used technology developed by a variety of companies, including RYLTI.

Marquez discusses the results of a recent survey about how elements of the industry are reacting to a post remote-work world.

The bill would remove financial for genetic testing determined to be clinically appropriate.

Both products are designed to help radiologists.

The conversation took place during the Veeva R&D Summit in Boston.

Veltre will develop the company’s media and digital strategy.

Alexander continues her conversation, touching on topics like disinformation and diversity in clinical trials.

Alexander discusses the broader implications the case could have on FDA's ability to regulate the industry.
The move will bring Landos’ lead drug, NX-13, under AbbVie’s umbrella.
The shortage is due to a reported manufacturing interruption.
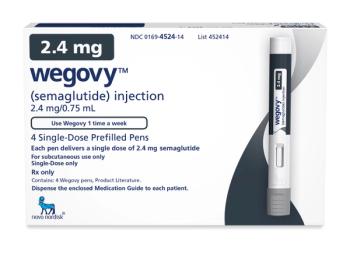
The drug was recently cleared to treat cardiovascular risks, making it eligible for Medicare Part D.

The agency released guidelines for vaccinations guidelines for children as young as 6 months old.

Cold Spring Harbor Laboratory details how the new tool will help reduce common barriers to trial participation.

Valder Curran discusses recent developments with the IRA.

The technology platform won the award for its work using AI to improve precision medicine.

The company will see the completion of its projects about a year earlier than expected.