
Accessing the latest cancer drugs always seem to be controversial. Not just because of the money but also because of the complexities of accessing treatments during their development.

Accessing the latest cancer drugs always seem to be controversial. Not just because of the money but also because of the complexities of accessing treatments during their development.

The Physicians Payment Sunshine Act, more formally referred to as Open Payments, has been in place for almost 18 months. It is only in the next few months, however, that the implications of the law and its contentious requirements will start to be fully realised. Michael Christel reports.

In the UK, Value-Based Pricing (VBP) has morphed into Value-Based Assessment (VBA), with the National Institute for Health and Care Excellence (NICE) tasked with taking the loose policy concepts (established as far back as 2010) and actually implementing them.

The Pharmaceutical Price Regulation Scheme (PPRS) is a long-standing scheme where the pharmaceutical industry, represented by the Association of the British Pharmaceutical Industry (ABPI), negotiates with the Department of Health (DH) to agree how to indirectly manage the price of branded medicines.

The first rule of effective communications - whether speaking to a beloved offspring or an important customer - is to know your audience.

One piece of fall-out from the recent Hobby Lobby decision by the U.S. Supreme Court is to generate talk of Republicans backing development of a nonprescription contraceptive pill.
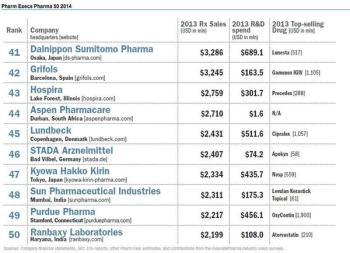
The vigorous return of M&A activity this year brings us back to the question: is more size and scalable efficiencies the best solution to the declining market power of pharmaceuticals in an endlessly restructuring healthcare system?

Is the FDA doing enough to incorporate the patient perspective in the drug review process? This was the key question considered at a panel of regulatory experts held at last month’s annual BIO International Convention in San Diego.

Pharmaceutical companies have begun using social media for business needs and promotion, yet have been hesitant to become too engaged since the FDA’s stance on social media activity remained unclear.

The Drug Information Association (DIA), one of the largest non-profit organizations supporting clinical research and drug development, held its 2014 annual meeting in San Diego last week. Applied Clinical Trials’ Moe Alsumidaie looks at three of the key themes from this year’s meeting: incorporating advocacy groups and patient voices in clinical research, breakthrough research applications, and new data collection methodologies.

FDA issued a warning letter to GlaxoSmithKline Biologicals (GSK), North America on June 12, 2014 for deviations from cCGMP requirements found during an inspection from Mar. 31 to Apr. 9, 2014 of its influenza vaccine manufacturing facility in Quebec.

The medicines business is all about data. Drug discovery relies on the meticulous interpretation of experimental results; clinical trials involve carefully analysing streams of information relating to patient response; even physician decision-making around the most appropriate therapies is becoming more and more data driven.
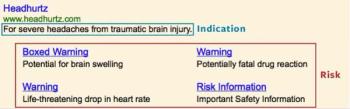
Earlier this year, FDA promised three sets of guidelines to clarify using social media for pharma marketing. Last week it delivered two of them
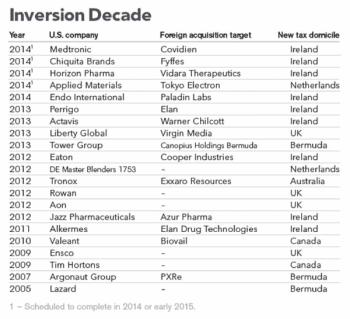
As I read the latest reports of yet another big US Rx firm, this time AbbVie, trying to pull off a “tax inversion” takeover of the Irish-based Shire, I couldn’t help but wonder, what the heck is going on here?

Do I have the only e-mail inbox clogged with more and more content about content marketing?

While at DIA’s 50th Annual Meeting, we got the chance to speak with Nancy Dreyer, chief of scientific affairs and senior VP at Quintiles Outcome, the CRO’s real-world and late-phase research division, about the current tide for comparative effectiveness research (CER).

Andy Bender and Geert van Gansewinkel outline eight lessons for executing a soft change program for enhancing compliance effectiveness within European life sciences organizations.

AbbVie’s recent $54 billion acquisition of Shire and subsequent relocation to Ireland will mean substantial tax savings for the US pharmaceutical giant, according to analysts at research and consulting firm GlobalData.

The pharma, medical and biotech industry is the leading sector for M&A activity this year, Mergermarket‘s Q2 M&A stats reveal.

For pharma companies, entering the social media scene is not as simple as just creating a Facebook page or learning how to tweet in 140 characters or less.

The UK government pledged late last year that it would double funding for dementia research by 2025 (from £66m [US$112m] in 2015 to £122m [US$207m]), but today Prime Minister David Cameron spoke on the subject again in light of expert warnings that little progress is being made.

At the annual meeting of the Food and Drug Law Institute (FDLI) this year, FDA commissioner Margaret Hamburg concluded her keynote address by describing a “dramatically changing global marketplace” and its “huge implications” for FDA’s ability to ensure the safety and quality of products manufactured elsewhere.

Big Pharma employment dropped by 3 percent in the decade 2003–2013, allaying fears that industry consolidation and restructuring would lead to significantly reduced headcounts and payrolls, a report by EP Vantage reveals.

A new report from GlobalData states that the increase of biosimilars will have a negative impact on the biologics market after 2019.

Biopharmaceutical drug discovery companies have progressively tailored their pipelines to specialty therapeutic areas and smaller patient populations.

When Audre McDonald accepted her sixth Tony Award (theater’s version of the Academy Awards) this month in New York City.

The European Medicine Agency’s Pharmacovigilance and Risk Assessment Committee (PRAC) has started a review to evaluate the cardiovascular risks with systemic ibuprofen medicines.
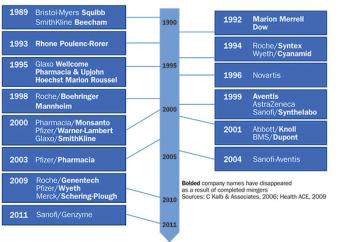
The major pressures hindering pharmaceutical industry success have not changed-payer constraints on drug costs, R&D productivity.

The future of GSK’s Tykerb (lapatinib) in the breast cancer market is unclear, after the combination of Tykerb and Herceptin in the large Phase III trial.

Brunei is harnessing its rich biodiversity and the growing halal market in a bid to develop its pharmaceutical sector.