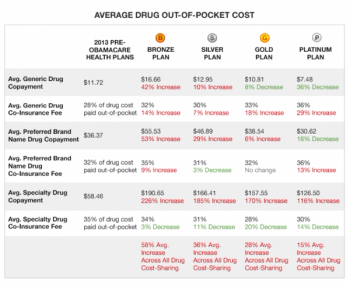
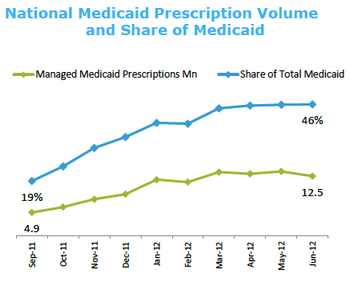
The Department of Veterans Affairs and other California panels’ solution to the new and extremely costly hepatitis (Hep) C drugs (Sovaldi and Olysio) is to reserve them for the patients with advanced liver diseases, including those awaiting transplants.