
They say the road to hell is paved with good intentions.

Pressure is mounting on European legislators to introduce tighter regulations at both the European Union (EU) and national levels on the potentially harmful impact of pharmaceuticals on the environment.

The recent geopolitical developments in Ukraine have seriously influenced the conditions and the environment for clinical trials in Ukraine and in Russia.
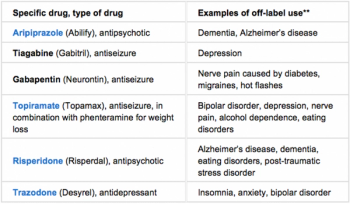
Years ago, while sitting in an Rx company lunch room, I was listening to one of the company’s top sales reps discuss the commercial progress a recently launched prescription was making.

Speaking at the 2014 New York BIO conference, FDA Commissioner, Dr. Margaret Hamburg, addressed the pressing issues affecting the biopharmaceutical industry and the FDA

Dealmaking lessons from history's storyboard.

Therapeutic specialization, competitive differentiation, and a finely-tailored value proposition are creating a new drug world of bespoke market niches-and infinite future possibilities for the best of this year’s Pharma 50.

Among the many announcements Apple made earlier this month at its annual World Wide Developer’s Conference, one was of particular interest to the healthcare industry.

The Rx360 pharmaceutical supply chain consortium celebrated its first five years with an anniversary conference on June 5 in Washington, D.C.

The UK’s National Institute for Health and Care Excellence (NICE) set out their proposals for value-based assessment (VBA) in March 2014.

Industry investment in pharma sales force and marketing channels remained “flat” in 2013 at just under €85 billion constant US dollars.

The European Federation of Pharmaceutical Industries and Associations (EFPIA) yesterday launched what it called “a landmark paper” outlining steps towards an integrated strategy for the life sciences sector in Europe.

While at the 2014 New York BIO Conference (NYBIO), Applied Clinical Trials‘ Moe Alsumidaie spoke to Nathan Tinker, Executive Director at the New York Biotechnology Association.

Private equity and venture capital (PEVC) deal activity decreased from 1063 in 2010 to 480 in 2013, according to a new Frost & Sullivan report.

Eli Lilly and Company has announced it will begin sharing its clinical trial data with scientific researchers through www.clinicalstudydatarequest.com.

There is more collaboration than ever between payers and providers in the drive to provide high-quality.

One of EMA’s key objectives this year is improvements in dealing with the “causes and impact of shortages of human medicines caused by GMP non-compliance and quality defects”.

A European bid to impose additional limits on research involving human embryos has been defeated.

I’ve been lucky enough to get a sneak preview of Across Health’s 2014 Multichannel Barometer report.

The Obama administration received a substantial amount of good news last month.

Vietnam’s pharma market in Vietnam is set to increase by $5 billion over the next six years.

Turkey is remains a key destination for foreign investment, and the next five years should see renewed interest from pharma multinationals, says a new report by CPhI Worldwide.

Effective clinical trial management depends on accurate and unbiased performance measurement.

FDA, in partnership with other federal and international agencies, has taken action against websites that sell potentially dangerous, unapproved prescription drugs to US consumers.
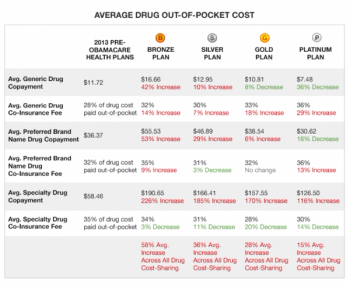
We are now approaching the six month point in the implementation of the new Obamacare program. It’s senseless to go into the good, bad and ugly of this new program.

In today’s pharmaceutical industry, it seems there are nearly as many communication channels as there are instruments in the Philharmonic.

Pharm Exec’s Brand of the Year selections from the last few years are still alive and kicking, although some have aged more gracefully than others.

Technology offers the promise of engaging patients on a level never seen before.

In the last three decades, MNCs have contributed greatly to the transfer of knowledge to generic companies in areas such as technology,

Every day, your competitors are doing everything within their power to steal your customers.