Joint venture is expected to leverage Astellas' expertise in cell therapy R&D and Yaskawa’s robotics technology to enhance accuracy, reproducibility, and efficiency in cell manufacturing.

Joint venture is expected to leverage Astellas' expertise in cell therapy R&D and Yaskawa’s robotics technology to enhance accuracy, reproducibility, and efficiency in cell manufacturing.

The latest news for pharma industry insiders.

In this Pharmaceutical Executive Video Interview, Peter Ax, Founder & CEO of UpScriptHealth, describes how his company's recent partnership with Pfizer is impacting drug access.

The acquisition is expected to enhance Jazz's oncology portfolio by adding dordaviprone, a novel small molecule therapy in development for H3 K27M-mutant diffuse glioma.
Submission is supported by positive results from the Phase III REGENCY trial, which demonstrated that nearly half of patients with lupus nephritis who received Gazyva/Gazyvaro plus standard therapy achieved a complete renal response.

The latest news for pharma industry insiders.
New indication for Tevimbra in combination with platinum-containing chemotherapy as a first-line treatment addresses an unmet need for adults with unresectable or metastatic esophageal squamous cell carcinoma whose tumors express PD-L1.
Stoboclo, a biosimilar to Prolia, is indicated for postmenopausal women and men at high risk of fracture, while Osenvelt, a biosimilar to Xgeva, is indicated for preventing skeletal-related events in patients with multiple myeloma and bone metastases from solid tumors.

Aliya Omer, VP, US franchise head, breast cancer, AstraZeneca, discusses how results of the trial compare with other treatments for gBRCAm HER2-negative high-risk early breast cancer.

The latest news for pharma industry insiders.
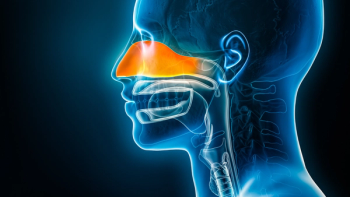
In the SWIFT and ANCHOR trials, depemokimab demonstrated statistically significant reductions in nasal polyp size, obstruction, and asthma exacerbations compared to placebo.

Under terms of the deal, Gubra will receive an initial $350 million payment and could earn up to $1.875 billion more in development and sales milestones for the potential weight loss drug.

Ron Lanton, Partner, Lanton Law, discusses the legal and industry challenges posed by recent National Institute of Health budget cuts, diversity bans in clinical trials, and pharmaceutical supply chain disruptions due to tariffs.

The latest news for pharma industry insiders.

Three of the new sites will focus primarily on producing ingredients for domestic usage.
FP008 is designed for patients with solid tumors that have not responded to existing immunotherapies targeting PD-1.

Results from a Phase III study in pediatric patients demonstrated that Odactra reduced the total combined rhinitis score by 22% compared to placebo.
A monthly roundup of business and people news in the pharmaceutical industry.

In this Pharmaceutical Executive Video Interview, Peter Ax, Founder & CEO of UpScriptHealth, addresses how tariffs will affect pharmaceutical costs and which drug categories will be most affected.

Results from a Phase III registrational study suggest that ecopipam has potential as a first-in-class dopamine-1 receptor antagonist for patients with Tourette syndrome.

The latest news for pharma industry insiders.

The Phase III SERENA-6 trial is the first global trial to use a circulating tumor DNA-guided approach to detect endocrine resistance before disease progression in HR-positive, HER2-negative advanced breast cancer.

Daniel Vitt, CEO, Immunic Therapeutics, discusses how the company is positioning itself to lead in the area of gastrointestinal diseases.
The popular medications may still suffer from local shortages, but FDA is confident that the national supply will remain stable.

The latest news for pharma industry insiders.

The application leverages existing clinical data for Uzedy along with prior FDA findings on the safety of Udezy in patients with bipolar I disorder.

The funding, led by existing and new investors, is expected to support Eikon’s efforts to integrate advanced computing, automation, and data science that drives innovation in drug development.

Daniel Vitt, CEO, Immunic Therapeutics, discusses how the safety and tolerabilities results from Phase I trials for IMU-856 influences further studies for gastrointestinal diseases moving forward.

In this Pharmaceutical Executive video interview, Jen Butler, Chief Commercial Officer of Pleio, discusses the significant threat of misinformation to public health and how emotional appeal plays a part.

The latest news for pharma industry insiders.