
The campaign is launching alongside positive news for the company’s Phase 3 trials for a GBS treatment.

The campaign is launching alongside positive news for the company’s Phase 3 trials for a GBS treatment.
In the Phase III DeLLphi-304 trial, patients with small cell lung cancer administered Imdelltra achieved a statistically significant and clinically meaningful improvement in overall survival compared to standard-of-care chemotherapy.

In the Phase III ODYSSEY-HCM trial, patients treated with Camzyos for symptomatic non-obstructive hypertrophic cardiomyopathy did not show significant improvements based on Kansas City Cardiomyopathy Questionnaire – Clinical Summary Scores and peak oxygen consumption.

Nigel McCracken, chief operating officer, Virax Biolabs, discusses results from new data on the role of T cell dysfunction in post-acute infection syndromes.

Despite meeting key pharmacokinetic goals, a potential case of drug-induced liver injury led Pfizer to conclude that danuglipron’s risk-benefit profile did not support further development for chronic weight management.
Odylia Therapeutics event brings together leaders across biotech, pharma, venture capital, patient advocacy, and research sectors to address the financial and structural barriers that impede rare disease drug development.
Approval of Opdivo plus Yervoy combination was based on results from the Phase III CheckMate-9DW trial, which demonstrated significantly improved overall survival and overall response rate in patients with unresectable or metastatic hepatocellular carcinoma.

Commerce Secretary Lutnick discussed the state of the tariffs in the wake of President Trump’s comments on the pharmaceutical and electronics industries.

The latest news for pharma industry insiders.

Investment is expected to bring approximately 1,000 new jobs at Novartis and 4,000 additional roles across the United States.
Full approval of Vitrakvi was based on results from three clinical trials in patients with unresectable or metastatic NTRK fusion-positive solid tumors.
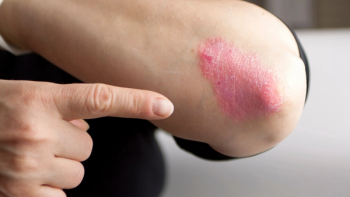
In the Phase III ICONIC-LEAD trial, 75% of adolescents with moderate-to-severe plaque psoriasis treated with icotrokinra achieved completely clear skin by week 24.

The latest news for pharma industry insiders.

Financing of Merida Biosciences was backed by Bain Capital Life Sciences, BVF Partners and Third Rock Ventures, with participation from multiple other investors.

Davidi Vortman, CEO, UltraSight, discusses how AI-powered heart scan technology can help high-risk cardiovascular disease patients.

The latest news for pharma industry insiders.

The company announced a new CFO along with the creation of a new role, chief medical officer.

PharmaLogic states that the acquisition of Agilera Pharma AS will place the company in a unique global role in radiopharmaceutical production and distribution.
Approval of the Opdivo plus Yervoy combination regimen was based on results from the Phase III CheckMate-8HW trial, which was the largest immunotherapy study in patients with previously untreated, unresectable, or metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer.

Brimochol PF, a combination of brimonidine and carbachol, was found to enhance depth of focus and visual acuity in clinical trials among patients with presbyopia.

The latest news for pharma industry insiders.

The survey also suggests that Medicaid is broadly popular amongst all Americans, regardless of political affiliation.

“We’re going to tariff our pharmaceuticals, and once we do that, they are going to come rushing back into our country," President Donald J. Trump said during a Tuesday night dinner in Washington.
Results from Phase II studies demonstrated that rilzabrutinib showed clinically meaningful outcomes in patients with warm autoimmune hemolytic anemia and IgG4-related disease.

The latest news for pharma industry insiders.

Family caregivers with both children and elderly family members to care for are reporting higher levels of burnout and mental health issues.

Results from the Phase III SPACE trial show that Ajovy significantly reduced monthly migraines in pediatric patients between six and 17 years of age.

Enrollment for the Phase II trial follows FDA clearance of SPG302 Investigational New Drug application for schizophrenia.

The latest news for pharma industry insiders.

Muscle loss is a serious issue for a number of patients, including those on GLP-1s.