The I LUVY YA for The Long Haul campaign features stories about the emotional impact of the disease.

The I LUVY YA for The Long Haul campaign features stories about the emotional impact of the disease.

Eli Lilly CEO David Ricks to the BBC: “It feels like it'll be hard to come back from here."

The latest news for pharma industry insiders.
Approval was based on results from the Phase III ALIGN study, which demonstrated a significant reduction in proteinuria at 36 weeks in patients with primary immunoglobulin A nephropathy.
Results from the Phase III ZENITH trial show that Winrevair lowered the risk of all-cause death, lung transplantation, and pulmonary arterial hypertension-related hospitalization by 76%.

Results from the SOLARIS trial survey showed that over 92% of patients, 87% of nurses, and 72% of physicians expressed satisfaction with TEV-'749’s initiation regimen, dosing schedule, and overall treatment experience.

The line-up consists of supplements to meet the health needs to patients taking GLP-1 medications for weight loss.

The latest news for pharma industry insiders.
BIIB080 marks the first antisense oligonucleotide targeting tau to enter clinical development for the treatment of Alzheimer disease.

Charles River Laboratories will work directly with the platform.

The latest news for pharma industry insiders.

Matthew Yelovich, partner at Cleary Gottlieb, discusses the appeals of Elizabeth Holmes and Ramesh "Sunny" Balwani and the Ninth Circuit's recent affirmation of their convictions.
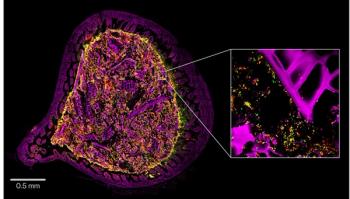
The platform can reportedly outperform traditional methods.
In the SOUL trial, Rybelsus produced a 14% risk reduction of major adverse cardiovascular events in patients with type 2 diabetes and cardiovascular disease and/or chronic kidney disease.
The trial size adjustment, along with protocol modifications and the addition of higher-enrollment sites, is expected to facilitate completion of the NEPHRO CRRT study of Niyad in patients undergoing renal replacement therapy by the end of 2025.

LillyDirect will help provide services to millions of patients who have yet to be formally diagnosed with the disease.

The latest news for pharma industry insiders.
Qfitlia is the first FDA-approved antithrombin-lowering therapy indicated for routine prophylaxis to prevent or reduce bleeding episodes in patients over 12 years of age with hemophilia A or B.

Approval of Imfinzi marks the first and only perioperative immunotherapy indicated for muscle-invasive bladder cancer.

A monthly roundup of business and people news in the pharmaceutical industry.

The latest news for pharma industry insiders.
Results from the COCOON study showed that Rybrevant plus Lacluze reduced grade 2 or higher dermatologic events by 50% compared to standard care in patients with epidermal growth factor receptor-mutated non-small cell lung cancer.
Based on additional results from the Phase III PSMAfore clinical trial, the therapy is now approved for use prior to chemotherapy in PSMA-positive metastatic castration-resistant prostate cancer.
Under terms of the deal, Novo Nordisk will obtain global rights to develop, manufacture, and commercialize LX9851 for obesity and metabolic disorders.

The high-stakes nature of cancer care, coupled with rapid scientific advancements and increasing payer and regulatory pressures, demands a specialized approach beyond traditional market access strategies.

The latest news for pharma industry insiders.
As part of the Fast Track designation, Sanofi is set to launch a Phase I/II trial to evaluate the immunogenicity and safety of its novel mRNA vaccine in preventing chlamydia.

Approval of Vykat XR marks the first treatment indicated for hyperphagia in patients with Prader-Willi syndrome.

The Healthwell Foundation published research that questioned over 1,500 Americans about their healthcare status.

The latest news for pharma industry insiders.