Today's FDA approval amends a previously granted accelerated approval for Balversa (erdafitinib) to treat patients with metastatic urothelial carcinoma whose tumors harbor FGFR3 or FGFR2 alterations following prior platinum-based chemotherapy.

Today's FDA approval amends a previously granted accelerated approval for Balversa (erdafitinib) to treat patients with metastatic urothelial carcinoma whose tumors harbor FGFR3 or FGFR2 alterations following prior platinum-based chemotherapy.
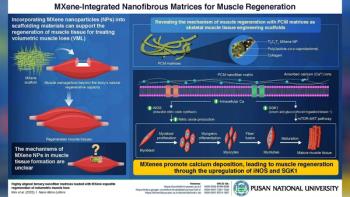
According to the university, new nanotech scaffolding can be used to support tissue growth.
Lutathera plus long-acting release octreotide lowered the risk of disease progression or death by 72% in patients with somatostatin receptor-positive well-differentiated grade 2/3 advanced gastroenteropancreatic neuroendocrine tumors.

In an interview with Nicholas Saraceno, Bill Roth, General Manager, Managing Partner, Blue Fin Group, discusses the changing role of PBMs and recent price structure overhauls.

Will the FDA ultimately step in and mandate a credible effort to eradicate the mindset that long-term adherence is not achievable?
Novocure’s tumor-treating fields plus standard-of-care therapies is under evaluation in patients with non-small cell lung cancer following disease progression on or after platinum-based treatment.

Sun Pharmaceutical Industries will purchase Taro's outstanding shares for $43 each, which will total $348 million.
Pivmecillinam has already been approved in Europe to treat uncomplicated urinary tract infections.
NX-5948 is under evaluation for for adults with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma following at least two lines of therapy that includes a BTK inhibitor and a BCL2 inhibitor.

In an interview with Nicholas Saraceno, Bill Roth, General Manager, Managing Partner, Blue Fin Group, discusses changes that will come with the pharma giant's new platform.
Bloomlife’s MFM-Pro is a prescription-based wearable device that can track the maternal and fetal heart rate.

HyQvia is the only subcutaneous immune globulin infusion that can be administered once per month.

Researchers believe low numbers in the use of direct-to-consumer healthcare services could rapidly trend up.
Vertex Pharmaceuticals' and CRISPR Therapeutics’ Cas9 therapy Casgevy approved as a one-time treatment for transfusion-dependent beta thalassemia on the heels of its approval last month for sickle cell disease.

Generative artificial intelligence, key new product launches, and the evolution of the go-to-market model are among key trends to watch in the year ahead.

Company displayed findings at the 2024 Winter Clinical Dermatology Conference in Hawaii demonstrating significant improvements in atopic dermatitis and seborrheic dermatitis.

New analysis for 2023 shows signs of post-pandemic recovery; breast cancer remains most studied disease area.

Soaring healthcare costs show little sign of slowing in 2024, with concerns growing about the affordability of medications and medical services.

Study highlights how AI can identify trends and connections that can predict how new patients might respond to therapies.
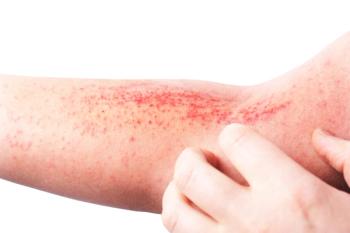
Dupixent is an IL-4 receptor alpha antagonist that has been approved for the treatment of adults with moderate-to-severe atopic dermatitis, as well as asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis.

Roots Analysis report suggests increase can be attributed to the growing demand for advanced therapies and biologics.

Each program strengthens the pharma value chain, impacting the core areas of clinical development, supply chain, and manufacturing and commercial engagement.

The FDA issued the 39th overall approval for Keytruda (pembrolizumab), with the latest in combination with chemoradiation for the treatment of patients with FIGO 2014 stage III to IVA cervical cancer.

Ginkgo Bioworks announces projected total revenue of $250–$260 million for 2023 for large scale data generation and artificial intelligence for biopharma R&D.

Although medications such as Mounjaro and Zepbound have shown efficacy in helping individuals lose weight, there are not yet enough results to fully support these claims.
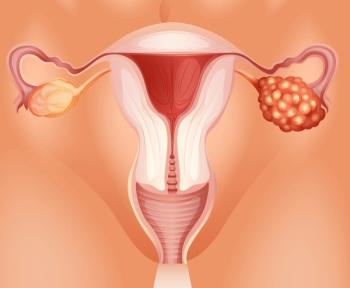
RC88 is under evaluation for the treatment of patients with platinum-resistant recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer.
Measuring the relationship between a product’s reimbursement position and performance begins with gathering key data points for as many products as possible.
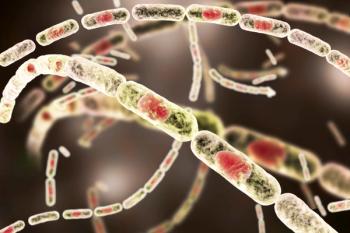
The BioThrax vaccine is indicated for active immunization to prevent anthrax disease caused by Bacillus anthracis in individuals aged 18 through 65 years.
Axon Therapy uses magnetic peripheral nerve stimulation to deliver a non-invasive treatment for chronic pain related to nerve damage caused by diabetes.
If the FDA approves the abbreviated new drug application for Lutetium Lu 177 Dotatate, the drug will be eligible for 180 days of generic marketing exclusivity in the United States for the treatment of somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors.