
This acquisition will add asthma treatment A1O-001 to GSK’s portfolio.

The Federal Trade Commission and Department of Health and Human Services are examining the practices of group purchasing organizations and drug wholesalers and the role they may play in triggering shortages of generic medications.

Lymphir is an immunotherapy under evaluation for the treatment of patients with relapsed/refractory cutaneous T-cell lymphoma following at least one previous line of systemic therapy.
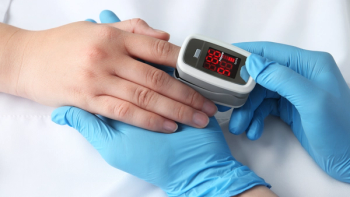
The MightySat Fingertip Pulse Oximeter is reportedly the first pulse oximeter available without a prescription.
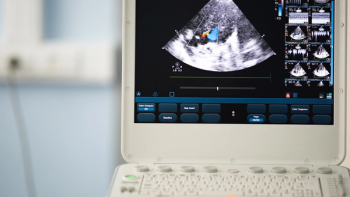
Device designed to address tricuspid regurgitation through a vein in the leg.

The campaign, that started in 2012, is back with seven new faces—many of which included messaging about the harms of menthol cigarettes.

Treatment aims to reduce the risk of finger or toe amputation due to frostbite.
Augtyro is a potential best-in-class tyrosine kinase inhibitor that targets ROS1- or NTRK-positive locally advanced or metastatic solid tumors.
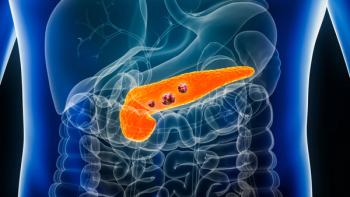
Pancreatic adenocarcinoma is the most common form of pancreatic cancer, with more than 60,000 diagnoses per year in the United States.
Researchers find that a thorough evaluation of drug spending and value can lead to a better allocation of healthcare resources.

Roche halts agreement with Repare Therapeutics to develop and commercialize camonsertib just weeks after triggering a $40 million milestone payment when the first patient was dosed with the novel cancer drug.
BXCL701 is an oral innate immune activator being investigated in combination with Keytruda (pembrolizumab) in patients with metastatic small cell neuroendocrine prostate cancer.

In this video interview with Pharm Exec Associate Editor Miranda Schmalfuhs, Dr. Jo Varshney, CEO & Founder of VeriSIMLife, discusses how artificial intelligence and machine learning can assist in the drug shortage problem.
In clinical trials, bepirovirsen showed the potential to address a significant unmet medical need for patients with chronic hepatitis B by reducing hepatitis B surface antigen levels and HBV DNA.
Takeda expects Eohilia single-dose stick packs will be available by the end of February for patients aged 11 years and older with eosinophilic esophagitis.

The FDA granted accelerated approval to Elahere in November 2022 for adults with folate receptor-alpha-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.

The use of artificial intelligence (AI) in drug development has been hampered by issues that have caused a gap between AI’s potential and its full utilization in this space.
Joint venture aims to submit an Investigational New Drug application to the FDA by early next year for treatment that targets solid tumors.

Novel oral orexin receptor 2 agonist produced statistically significant and clinically meaningful improvements in wakefulness compared with placebo in patients with narcolepsy type 1.

In an interview with Pharm Exec Associate Editor Don Tracy, Jonathan Miller, General Counsel, US Hemp Roundtable, discusses recent efforts that urge the House Energy and Commerce Committee to expedite an FDA hearing on the hemp market.

Researchers evaluate a potential association between the use of phosphodiesterase type 5 inhibitors for erectile dysfunction and a reduced risk of Alzheimer disease.
JNJ-2113, an oral IL-23–receptor antagonist peptide, showed consistency across clinician and patient-reported outcomes in patients with moderate-to-severe plaque psoriasis.

Data from the ENHANCE-3 trial of magrolimab in combination with azacitidine plus Venclexta showed futility and an increased risk of death in patients with acute myeloid leukemia.

As the market for biosimilars expands, a nuanced approach is needed to balance cost considerations with patient safety and pharmacovigilance efforts.
The biopharma company’s Phase 3 trial for its acoramidis product generated statistically significant results.

The Maryland facility will create cell therapies for use in future cancer trials.

Results from the Phase III CheckMate -77T trial show that Opdivo (nivolumab) plus chemotherapy followed by surgery and adjuvant Opdivo produced statistically significant and clinically meaningful improvements in event-free survival in patients with resectable stage IIA to IIIB non-small cell lung cancer.
Vepdegestrant (ARV-471) is a noval PROTAC ER degrader found to harness the body’s own natural protein disposal system to eliminate disease-causing proteins.

The agreement will strengthen Novartis’ oncology pipeline.
Cancer & Work: Acting Together guarantees job security and full salary continuation for up to twelve months for Sanofi employees and family members diagnosed with critical illnesses.