
Arthritis, asthma, cancer, cardiovascular diseases, chronic kidney disease, COPD, depression, and diabetes reach the highest recorded levels since the America’s Health Rankings Annual Report began tracking them in 1990.

Arthritis, asthma, cancer, cardiovascular diseases, chronic kidney disease, COPD, depression, and diabetes reach the highest recorded levels since the America’s Health Rankings Annual Report began tracking them in 1990.
Migraine with comorbid obesity have been found to cause high levels of disability and are both more common in female patients.
Trial of Keytruda (pembrolizumab) plus chemotherapy and maintenance Lynparza (olaparib; AstraZeneca, MSD) for the treatment of metastatic squamous non-small cell lung cancer stopped after data did not show a survival benefit.

UCB executive speaks on recent FDA approval of Bimzelx.

Experts from Hootsuite noted that the primary social media ROI concern for healthcare companies is the time and money it takes to maintain a multi-platform presence.

CVS Health announced a new pharmacy reimbursement model, called CVS CostVantage, which will define drug cost on the amount it pays for a drug, a set markup, and a fee for pharmacy services.

Fabhalta (iptacopan) is the first oral monotherapy approved by the FDA for the treatment of adult patients with paroxysmal nocturnal hemoglobinuria.
National Library of Medicine study emphasizes inadequacies in ensuring the safety and efficacy of over-the-counter dietary supplements.

An overview of the nine biggest developments to expect across the healthcare industry in the year ahead.

Jennifer Breheny Wallace, award-winning journalist and author, notes that C-Suite leaders should understand the financial risks of not taking action to address workplace burnout in 2024.

Patients with obesity are now able to access Zepbound (tirzepatide) with a prescription at retail and mail-order pharmacies across six dose strengths.

TAR-200 has a novel targeted releasing system for the treatment of patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer who are ineligible for bladder removal surgery.
The FDA assigned a PDUFA date of April 5, 2024 for Opdivo (nivolumab) plus cisplatin-based chemotherapy for the first-line treatment of adults with unresectable or metastatic urothelial carcinoma.
Pharma industry stakeholders must develop actionable roadmaps to prioritize the areas in which the use of generative artificial intelligence can create the greatest benefits.

JAMA study evaluates differences in coverage types and potential from 2016-2021.

Roche announces definitive merger agreement to acquire Carmot Therapeutics, Inc., which has an R&D portfolio that includes clinical stage subcutaneous and oral incretins for the treatment of patients with obesity, both with and without diabetes.
Jaypirca (pirtobrutinib) granted accelerated approval by the FDA for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma who were previously administered least two prior lines of therapy that included a BTK inhibitor and a BCL2 inhibitor.

Findings show that a considerable number of adults remain uneducated on how herpes zoster is triggered.

How to build a loyal audience of brand evangelists.

Artificial intelligence is making it quicker to get drug candidates to the clinic, but it isn’t addressing the fundamental need to marry the right candidate and the right target to the right disease.
The FDA previously granted accelerated approval to the Keytruda plus Padcev combination for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are ineligible for cisplatin-containing chemotherapy.
FDA to expedite review of zotatifin plus Faslodex (fulvestrant) and Verzenio (abemaciclib) as a second- or third-line treatment for patients with estrogen receptor–positive, human epidermal growth factor receptor 2-negative advanced or metastatic breast cancer whose disease progressed after treatment with endocrine therapy and a CDK4/6 inhibitor.

The use of real-world evidence can prevent pharma companies from performing studies based on flawed data.

Blenrep significantly extended the time to disease progression or death against existing care methods as a second-line treatment for relapsed or refractory multiple myeloma.

JAMA study investigates whether consuming energy drinks was associated with adverse pregnancy outcomes and whether caffeine consumption affects fetal-growth restriction.

AbbVie acquiring all outstanding shares of ImmunoGen, Inc. for $31.26 per share, valuing the company at a total equity value of approximately $10.1 billion.
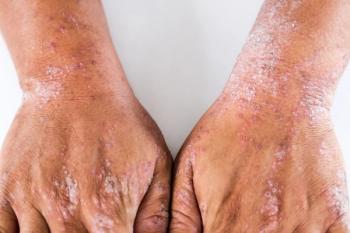
Supplemental new drug application for roflumilast cream 0.15% to treat atopic dermatitis in patients 6 years of age and older was assigned a Prescription Drug User Fee Act target action date of July 07, 2024.
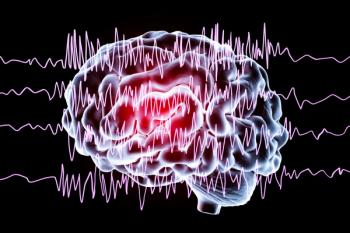
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a rare but serious and potentially fatal reaction linked to use of antiseizure medications.

KarXT (xanomeline-trospium) is currently in development to treat schizophrenia and psychosis related to Alzheimer disease.

Vivos becomes the first company to bring to market an alternative to continuous positive airway pressure (CPAP) or surgical neurostimulation implants for patients with severe OSA.