
As a European politician attacks abuses of the orphan drug scheme, a European report extols its virtues. Reflector asks: in Europe, does the right hand know what the left hand is doing?

As a European politician attacks abuses of the orphan drug scheme, a European report extols its virtues. Reflector asks: in Europe, does the right hand know what the left hand is doing?
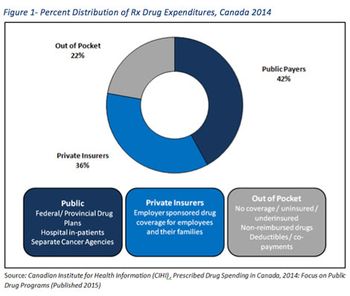
Arvind Mani and Sherry O' Quinn outline the prevalence of private payer PLAs in Canada.

Following the consultation on the future of England's Cancer Drug Fund, Leela Barham reviews what the stakeholders are thinking.

Whatever used to be wrong with the world of big Pharma could be fixed with a single tag phrase: emerging country markets. Most of the majors have invested heavily in this geographic segment, and the biggest of the big-companies like Novartis-now rely on it for more than a quarter of their global sales. Like all good things, however, there are shadows amidst the sunlight, and the task of turning volume sales into sustainable profits is getting harder.

Pharm Exec interviews Subhanu Saxena, CEO of India pharma giant Cipla, who discusses the longtime emerging market champion’s strategic plans to raise its geographic profile and secure the company’s future in the US.
In tandem with South Africa’s institutional advancements, driven by sweeping social and macroeconomic reforms, the pharma industry in the region is capitalizing on its core fundamentals: rising consumer disposable income, a positive demographic profile linked to an aging population, and a diverse disease burden requiring novel cures and treatments.

It’s easy to foresee controversy in 2016 as lagging global economic growth and a messy US political slugfest force the pharma industry’s feet to the fire. Can the industry reclaim a semblance of public approval by confronting its pricing demons and focusing on innovation and a fresh, patient-first message?

Julian Upton sits down with Subhanu Saxena for an update on Cipla's plans to leverage its historic visibility as an emerging market champion to the next level of global leadership in biopharma.

It's an obvious question for every pharmaceutical executive - and the question no-one dares to answer: what happens if the UK leaves the EU?

Kevin E. Noonan asks, what do the biosimilars provisions of the Trans-Pacific Partnership portend for global biologic drug development?

Telling signs from U.S., Russia, Latin America and UK indicate that governments are squeezing as much value as possible out of their spending on pills and procedures.

Will the UK learn from past efforts to champion innovation in the NHS? Leela Barham assesses the Phase 1 evaluation of its Innovation, Health and Wealth initiative.

Confronted by a debt crisis and a stagnating economy, the government of Puerto Rico, the world’s fifth-largest pharma manufacturing producer, has turned to the healthcare industry-and life sciences in particular-for new sources of economic growth.

A Pharm Exec conversation with AmerisourceBergen CEO Steve Collis

In the last year regulatory authorities sharpened their focus on gathering more data and on achieving transparency and harmonization. Erick Gaussens reviews 2015 and looks at companies’ need to respond to converging demands in 2016.

The Parliamentary Assembly of the Council of Europe is the latest to clamber aboard the well-filled bandwagon now rumbling and rattling across Europe in an increasingly noisy debate over drug prices. Reflector reports.

The much awaited consultation on a ‘new’ Cancer Drugs Fund (CDF) in England has finally emerged. Leela Barham reports.

The new interim report on the UK's Accelerated Access Review, which aims to speed up access to innovation in the NHS, is lacking in detail, writes Leela Barham.

Ebola showed that when innovation is truly integrated, we can enter into unknown territories and bring new innovations with us, writes Neil Saward.

In Pharm Exec’s 2013 end-of-year supply chain roundup, we began with a three-word vision of the immediate future that left little room for ambiguity: “Serialization is coming.” With the impending laws regarding “track and trace” promising to alter the way pharmaceuticals are packaged and shipped, we outlined how global pharma was gearing up to deal with the effects of serialization, and how companies needed to review their own internal practices and those of their outsourcing partners, as the need for technology solutions for both sides of the outsourc

Some of the radical thinking on healthcare spending from a new EC specialist group offers robust food for thought, writes Reflector.

Having reached the end of a decade of marked transformation and overhaul to Turkey’s healthcare system, identifying and acting on the resulting trends, dynamics, and initiatives will be key for the Turkish pharma industry going forward.

AmerisourceBergen believes the power of community begins with talk that gets the blood flowing.

In the midst of a leadership vacuum at the European Medicines Agency, Pharm Exec talks with the organization’s top medical officer, Hans-Georg Eichler, about its potentially game-changing drug approval program-one designed to balance safety requirements with faster patient access to the strong science now emerging from industry labs.

No-one can say England's Accelerated Access Review, which aims to “ensure that the UK is the fastest place in the world for the design, development and widespread adoption of medical innovations" isn't aiming high, writes Leela Barham.