
With specialty companies getting smarter in applying their big data insights to product marketing, the true commercial potential of machine learning and predictive modeling may soon be within reach.

With specialty companies getting smarter in applying their big data insights to product marketing, the true commercial potential of machine learning and predictive modeling may soon be within reach.

An overview of Orphan Reinsurer and Benefit Managers groups, including potential challenges with the model and various model suggestions.

How pharma companies are leveraging natural language processing (NLP) for rapid and effective handling of unstructured text.
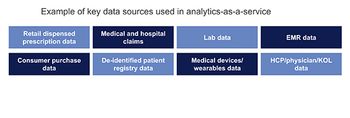
Analytics as a service can provide critical accelerators for the commercial transformation program at a life sciences organization, writes Himanshu Jain.

Benefit verification (BV) provides a detailed understanding of out of pocket costs for the prescribed treatment. Amy Jones details how AI is aiding the BV process.

While digital transformation in life sciences starts with digitization for compliance, it should be seen as a key lever of competitive advantage and success, writes Jaleel Shujath.

Artificial intelligence adoption by pharma companies in the UK demands a new, agile governance layer, write Tim Wright and Antony Bott.


If advanced technologies hold the key to at least some of the productivity issues R&D organizations need to overcome, Nicholas Lakin asks what are the best strategies and operating models for exploiting these aids to their fullest potential.

Traditional scientific search is broken. AI-supported smart searching is the answer, writes Jane Reed.

Ron Wince explains how Pragmatic AI allows organizations to start with the basics, create immediate business value and ROI and then increase adoption as their comfort, capabilities and confidence increases.

At this month's AMPLEXOR Life Sciences’ “Be The Expert” conference, Pharm Exec caught up with Steve Gens, who talked about the evolution of Regulatory Information Management (RIM) and offered some predictions for 2022.

Realistic, multisensory training devices can contribute to a drug’s reception in the eyes of both patients and healthcare practitioners, writes Paul Sullivan.

Ilyssa Levins talks to Pharm Exec about some of the regulatory, compliance and legal challenges posed by the digital health revolution.

From AI to Alexa, experts in specialty pharma ponder the possibilities for high tech and the patient journey.

Structured authoring is an approach that has eluded life sciences, limiting firms’ ability to transform routine regulatory processes. But this could all change, writes Romuald Braun.

Digital health is not at all about the app. It’s about the behavior of the individual patient, said Søren Smed Østergaard at this year's Digital Health World Congress in London.

Otsuka Pharmaceutical’s President and CEO of Development and Commercialization William Carson explains the initial growing pains the company went through with their technology company partner in the early development stages of the first U.S. FDA approved digital medicine system.

Right now, the smartest incumbents are channeling the forces of disruption to their own ends, leading digital transformation in order to avoid being left behind, writes Tony Owens.

William Carson, president & CEO of Otsuka Pharmaceutical’s development and commercialization, talks about the challenges facing the pharmaceutical industry when it comes to the massive amounts of data being collected.

Innovations in Big Data will have a lasting impact on care and present an opportunity for U.S. pharmaceutical companies, writes Nobuko Kobayashi.

Ashley Slavic outlines the preparations for the new EU data protection regulation, and what others can learn from the experience

The use of more targeted therapies is expanding as the public gains access to low-cost genetic testing, and more advanced computer systems are offering data from healthcare systems.

Meeting the stringent cloud compliance and regulatory requirements in pharma.