
Technology
Latest News


Looking at the balance of optimism and caution discussed at eyeforpharma Barcelona around pharma’s embrace of AI.

Three ways AR can bolster pharma commercialization strategies.


For companies that overcome the challenges, there is tremendous opportunity to drive sustainable growth through technology and analytics-driven business innovation, writes Vita Cassese.
Innovations in consumer genetic health are rapidly advancing-and pharma commercial thinking needs to catch up.

Adhering to new compliance legislation such as Identification of Medicinal Products (IDMP) can bring a pharma company benefits as well as challenges, writes Darren Cooper.

Rather than looking at what their peers are doing in digital health, pharma companies need to anticipate new challengers in the healthcare industry, writes Kal Patel.
This blog discusses the benefits of the pharma industry adopting the idea that media is data.

Are the technologies and assumptions at the heart of healthcare AI trustworthy?

This article discusses real-time data capture and analytics in clinical trials.

Danish Gupta discuss the current strengths and weaknesses of artificial intelligence applications in life sciences.

Using intelligent automation of content creation can turn master product data into business value, writes Siniša Belina.

mHealth devices and apps that continuously monitor patient symptoms and help with adherence also are likely to reshape medical practice – and with it the products pharma produce, writes Vicki Anastasi.

Five predictions on evolution of data and predictive insights that could be critical for pharma go-to-market strategies.

William King offers five predictions on evolution of data and predictive insights that could be critical for pharma go-to-market strategies.

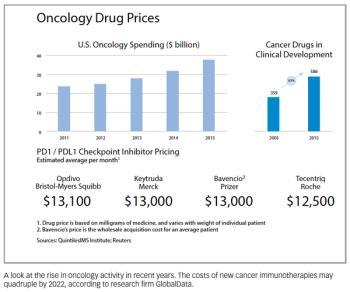
Sean McCarthy, President & CEO of CytomX, outlines the company’s journey from university startup to rising star of the immuno-oncology space.

In the search for effective and efficient ways to bridge the urban/rural divide, technological advances are offering more and more solutions.

FDA's approval of the first-ever digital medicine, while sparking legitimate concerns around ethics and trust, could signal improvements ahead to the much-chronicled woes in drug adherence and compliance.


Pharm Exec speaks to Prognos CEO Sundeep Bhan about how the company’s unique offering will reach beyond life sciences as the long-promised benefits of AI become reality.


Selecta Biosciences CEO Dr. Werner Cautreels talks about the company's innovative technology and how his entrepreneurial experiences in big pharma prepared him for the challenge of a new startup.

Today’s model requires a fourth partner-the systems integrator



