
Peter Kohut analyses the findings of a survey on how life sciences companies are capitalizing on opportunities to transform workload and data quality management using the latest smart technology.

Peter Kohut analyses the findings of a survey on how life sciences companies are capitalizing on opportunities to transform workload and data quality management using the latest smart technology.

The recent merger of Teledoc and Livongo into a $38 billion titan proves that the market sees opportunity in health tech. Could it be an inflection point in our race towards a digital-first care model, or is this an experiment ahead of its time?

EXUMA Biotech's Dr. Greg Frost talks about his work making CAR-T cell therapy readily accessible and affordable.

Their application for security and anti-counterfeiting purposes continues to evolve, especially in combination with digital technology.

When approached correctly, drug development in the rare disease space can be quite successful – especially when powerhouses across the industry join forces and leverage the power of data to help drive potential treatment options.

How companies are leveraging Real Word Evidence to evaluate therapies under real world conditions in a broader population at a lower cost than using Randomized Clinical Trials.

A new patient journey is emerging. Gilda Sala, Trista Bridges, and Rune Soelvsteen offer easy steps to future-proof your company’s approach.

Stephanie Hill looks at the efforts made by patient solutions providers in the UK to build and support services outside the hospital environment during the pandemic.

Priyanka Chaudhary and Vikram Shetty outline the current challenges and opportunities presented by e-labeling around the world.

Xavier Duburcq describes how pharma leaders can convert compliance from a cost center to a critical success factor.

A crisis like COVID-19 is not one event, but many interconnected crises. If we’re not constantly learning and adapting, we’re going to be facing a long, painful recovery. We need to modernize how we operate, writes Derek Choy.

With medical meetings and congresses going virtual in the face of COVID-19, how do pharma companies put together a strategic plan for a new format that meets goals while remaining sensitive to the broader environment?

Hunting for effective treatments, tools offer support in global fight.

Pharma companies may be in a unique position to support at-risk patients with chronic illnesses at this unusual time, particularly if they already have patient support programs in place with a patient services hub, writes Rebecca Cotton.

The need for analytics translators is not limited to data science. The adoption of all scientific or technical advances, including those in the healthcare industry, needs effective translators. So what does this mean for pharma marketing?

A centralized platform for assets supports the industry’s need for a greater volume of more diverse content, writes Callum Hawes.

Matthew Sussman explores strategies for facilitating access to the timely patient data and analysis needed for successful value-based agreements between manufacturers, payer and provider organizations in 2020 and beyond.

How exactly do pharma companies account for a gap between their hopes for a new drug and reality? Peter Harbin reports.

From lightweight ERP work, to break fix services, to infrastructure- how start-up life sciences organizations, or those new to the outsourcing model, are benefiting from the use of IT partners.
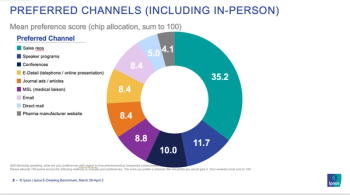
Over the coming months, HCPs and manufacturers will experiment with different approaches to e-detailing, in order to arrive at an approach that works for everyone, writes Scott Morano.
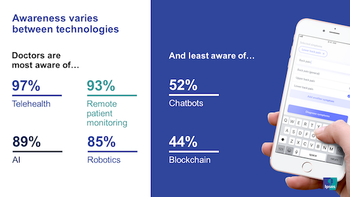
Before the COVID-19 outbreak, a number of doctors were starting to use digital and connected health but many remained hesitant. Now, this has all changed.

Steve Gens and Remco Munnik offer five best-practice tips for achieving a definitive, trusted regulatory and product information asset base capable of supporting intelligent innovation.

In safety & pharmacovigilance, the requirement to capture, sift and process real-world adverse event data is vast and growing all the time. Why then, writes John Price, is the discipline lagging in its application of smart technology?
Thriving in the new COVID-19 world requires five key ingredients, which can be leveraged with a transformative approach to digital adoption in the pharma space.

Grünenthal's Florent Edouard and Veeva's Jan van den Burg talks about how the COVID-19 pandemic is changing the way the industry reaches and engages with its customers.