
Exploring the challenge for pharma in adapting multichannel marketing strategies for the global stage, while trying to refine messages and communicate a brand identity to multiple local audiences.

Exploring the challenge for pharma in adapting multichannel marketing strategies for the global stage, while trying to refine messages and communicate a brand identity to multiple local audiences.

Amid concerns over a potential 'wild west' climate for data analytics in pharma, taking a long-term, proactive, and enterprise view of your company’s data may be the best way to keep your organization and reputation safe.

How to capitalize on the rise of omnichannel engagement in pharma and pursue myriad modes of message delivery in today’s digital world.

A look at the ongoing barriers and opportunities in building connections between HCPs, patients, and pharma through social media.

The key to success in a hypercompetitive marketplace is to create targeting plans that get ahead of tomorrow’s prescriptions, write Janardhan Vellore and Daniel Wetherill.

In 2020, AI will become pervasive in enterprise applications and embedded within specific commercial workflows, writes Paul Shawah.

Having invested heavily in new regulatory database systems, life sciences firms owe it to themselves to capitalize on the insights locked within those rich data assets, writes David Gwyn.

How successful commercial teams will balance the continued shift toward personalized medicine and the increased concentration on data privacy.

Goals for IDMP in Europe must not be diluted in 2020, if standardized medicinal product data is to be of tangible real-world benefit, says Remco Munnik.

Kellie Rademacher identifies some of the key questions to consider when choosing an AI partner.

Graham Francis sets out 5 critical business drivers for building a global enterprise labelling strategy over the year ahead.
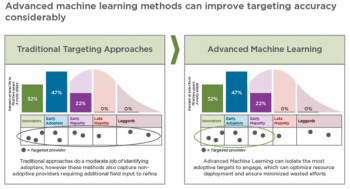
How machine learning can provide an on-demand portfolio-wide view of optimal provider targeting and related field deployment.

Organizations need to be correctly set up to deploy AI and machine learning. A new approach to processes and content is often required, write Jan van den Burg and Abid Rahman.

Aiforia Technologies CEO Kaisa Helminen talks about the benefits of pharma embracing digital technologies, especially AI-assisted image analysis in the field of histopathology.

Technology grabbing the attention of watchful industry

Amid new findings on today’s C-suite mood and approach to digital transformation, experts-in a panel discussion and wider examinations by Pharm Exec editors-explore the intersection of data and technology and the areas that must evolve to meet the digital health demands of tomorrow.

Despite the buzz, physicians have limited familiarity and understanding with the burgeoning category of nucleic acid-based therapies, writes Meghan Oates-Zalesky.

Most emerging pharma companies still struggle to amass the computing power and assemble the teams to make use of the vast amounts of data now available to them, write Beth Beghou and Patrick Lezark.

In comparison with other industries, the use of AI is modest in life sciences. However, even within life sciences, adoption of AI in regulated environments, such as in the R&D value chain, is further behind. Sivakumar Thiagarajan

Mike Kean and Billy Tamulynas discuss the key components of growth-ready technology for early-stage pharma companies and how to make optimal use of them.

Getting real about the R&D capabilities required to win in the gene therapy space.

AI has huge potential, but the key to ROI is in the application, writes Alan White.

With a growing abundance of clinical and health information now available and achievable, the need for industry to apply common structures and rules to interpret and act on this data is critical.

Realizing the full potential of enabling technologies such as AI and machine learning in measuring treatment value and outcomes.

In keeping pace with the digital transformation, pharma companies are doing pretty well with data and infrastructure, but a fundamental reshaping of operating models remains the biggest hurdle to moving forward.