
Part of the value of a drug comes from the supply chain that protects its integrity. There's a similar supply chain that preserves the value of drug information. And it needs help.

Part of the value of a drug comes from the supply chain that protects its integrity. There's a similar supply chain that preserves the value of drug information. And it needs help.

A review of the best ads of 2004--and the people that created them.

A recently released report claims pharma hides bad clinical trial results and over-promotes drugs. Tougher regs are being called for.

Cover Story
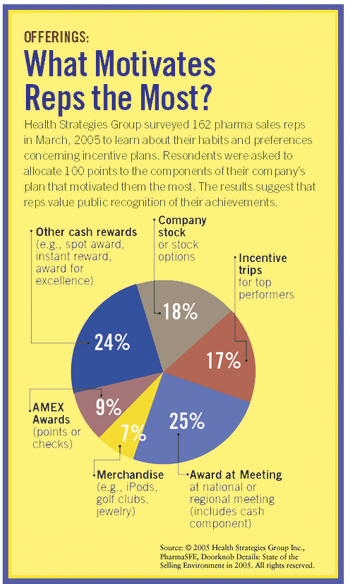
Employees are most attracted to products they might feel guilty about buying for themselves, but when they have points to redeem, they feel fine about splurging.

TOC

Post-approval studies, at many companies, do not have a consistent strategy that builds networks of investigators.

Conventional wisdom says that generics businesses are a drain on valuable resources. But these units are much less prone to the disastrous drug safety panics that Big Pharma has recently endured.
Recently developed technologies produce clinical quality cells in as few as12 days. Culturing cells in petri dishes can take a month or more.

Cover Story

Johnson & Johnson's pharmaceutical group builds team and relationship skills into performance evaluations. J&J's Robert Wills says, "Such skills are a built-in expectation. It's how people are supposed to do their job. Everyone who participates in an alliance is compensated for behaviors that contribute to mutual success."

OVERVIEW

Pharma companies in Europe believe that it already takes too long for new medicines to reach patients. Separate bodies for efficacy and safety will lead to further delays.

Pharm Exec talks to the newly appointed leaders of the industry's most influential advocacy organizations.

The LazarusEffect

The High Court ruled that Abbott should pay the agreed-on royalty of 5 percent rather than the 2 percent it's been paying. The difference may be worth $392 million

The 2005 Conference

Heading the WHO list are pandemic influenza and antibiotic-resistant bacterial infections. Together the two could kill millions.

This is the time of year to not only reflect on the past, but to ponder the future.Forget soothsayers, prophets, and fortune tellers' crystal balls. Inside are predictions from some of industry's key thought leaders.

Magic Molecules

China's large patient base and concentrated specialty hospitals make it easier to recruit patients for clinical trials in than in the United States. On average, companies can save from two to five months by conducting a Phase I study in China.

The British trade association says the cuts are unnecessary because drug prices have fallen in real terms by 10-15 percent during the last 10 years while the UK government's budget has remained steady.

Expanded coverage for the uninsured and Medicaid reform are low priorities at the White House.

Journalists try to tell both sides of a story. That's fine when there are two groups in conflict. But how do you tell the story right when the real conflict is between the two halves of one ambivalent opinion.

Pharm Exec's Pipeline Report is packed with 25 of the year's most eye-catching experimental drugs. What's their secret? No smoke or mirrors-just innovative science, therapeutic value, and good business sense.

If trial results never reach the public, researchers could waste time repeating lines of inquiry that have already proven unsuccessful.

Lifecycle management and line extensions helped the Percocet franchise generate steady annual growth, rising from $40 million in 1997 sales to $214 million in 2003, despite the fact that it had no patent protection.

Finger pointing has begun in earnest, as Congress tries to decide who ignored what warning, who silenced what staff scientist, and who broke what law. But recent events also raise real long-term issues for industry.
The global pain market will reach nearly $29.8 billion in 2008, of which $21.8 billion will come from the United States.

Reeve had done something that many of us who communicate about health issues try in vain to accomplish.