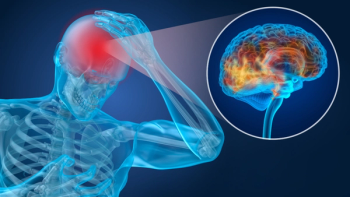
The concussion test, which shows results after 15 minutes, can help evaluate patients up to 24 hours after injury, company says.

The concussion test, which shows results after 15 minutes, can help evaluate patients up to 24 hours after injury, company says.

Approval would mark first HER2-targeted therapy for biliary tract cancer in the United States.

In this Pharmaceutical Executive video interview, Optum Rx clinical pharmacist, Arash Sadeghi, discusses upcoming advancements in the drug pipeline and trends shaping the future of drug development.

The two organizations will work together to advocate for better health outcomes for children and families in North Carolina.

The latest news for pharma industry insiders.

The network will work with DiRx and MakoRX.
Treatment approved in combination with ravulizumab or eculizumab, representing minority demographic still suffering after receiving C5 inhibitor therapy for extravascular haemolysis.

In this Pharmaceutical Executive video interview, Optum Rx clinical pharmacist, Arash Sadeghi, discusses developments for non-invasive diagnostics for liver disease and how such advancements could potentially impact the landscape of conditions like NASH.

In an interview with Pharm Exec Associate Editor Don Tracy, Fady Boctor, President, Chief Commercial Officer, Petros Pharmaceuticals provides his thoughts on what the future looks like when it comes to over-the-counter (OTC) contraceptives.

The latest news for pharma industry insiders.

Company says it remains committed to advancing research for immune-mediated diseases and intends to share trial data at a later date.

The news comes less than a year after Pfizer announced that IPG would handle this role.

The study used technology developed by a variety of companies, including RYLTI.
Phase II clinical study showed that children as young as six years of age with hepatitis B virus could benefit from treatment with Vemlidy.
Deal aims to commercialize XTX301 for treating advanced solid tumors by leveraging Xilio’s tumor-activated immuno-oncology therapies.

The latest news for pharma industry insiders.

The bill would remove financial for genetic testing determined to be clinically appropriate.

Both products are designed to help radiologists.
Approval of Vafsen (vadadustat) based on promising results from the INNO2VATE program and additional safety data from use in Japan in adults with chronic kidney disease.
Evolut FX+ transcatheter aortic valve replacement system design includes a larger coronary access window through a modified frame.

The benefits of adopting a humanized approach to healthcare provider marketing extend across the healthcare spectrum, enhancing engagement rates, brand loyalty, and marketing ROI, while also driving better outcomes for patients.

In an interview with Pharm Exec Associate Editor Don Tracy, Fady Boctor, President, Chief Commercial Officer, Petros Pharmaceuticals talks about the recent availability of Opill as an over-the-counter (OTC) contraceptive.

The latest news for pharma industry insiders.
Action marks the first FDA-approved blood screening test for malaria.
Winrevair is the first FDA-approved activin signaling inhibitor therapy for pulmonary arterial hypertension.

Veltre will develop the company’s media and digital strategy.

The latest news for pharma industry insiders.

Reportedly, the oral weight loss pill VK2735 showed promising signs of effectiveness and a tolerable safety profile.

In this Pharmaceutical Executive video interview, Optum Rx clinical pharmacist, Arash Sadeghi, explore mixed trial results impact the confidence in their potential real-world effectiveness and potential market adoption.
Deal includes the novel treatment CDR132L, which is currently in Phase II clinical trials for heart failure.