
Lynlee Brown discusses the decisions that companies must make to adjust to the tariffs while also ensuring the ongoing innovation continues.


Lynlee Brown discusses the decisions that companies must make to adjust to the tariffs while also ensuring the ongoing innovation continues.

Four tips for midsized companies when entering new global markets.

How to maximize market-entry performance for new pharma products in an increasingly competitive launch environment.

“We’re going to tariff our pharmaceuticals, and once we do that, they are going to come rushing back into our country," President Donald J. Trump said during a Tuesday night dinner in Washington.

The high-stakes nature of cancer care, coupled with rapid scientific advancements and increasing payer and regulatory pressures, demands a specialized approach beyond traditional market access strategies.

Kimberley Chiang, vice president of biopharma commercial solutions at CoverMyMeds, discusses how the industry leverages data and technology to identify and address affordability issues before they become a barrier to patient access.

A look at the current landscape and how young companies can set the stage for product launch.

HEOR scientists in the pharma industry must balance methodologic rigor with commercial expectations and timelines.
The recent shift in Health Economics and Outcomes Research functions across major pharmaceutical companies highlights a lack of understanding of its value.

In this Pharmaceutical Executive Video Interview, Peter Ax, Founder & CEO of UpScriptHealth, explores ways that digital health platforms can assist pharma companies seeking to maintain accessibility and manage cost pressures.

In this Pharmaceutical Executive Video Interview, Peter Ax, Founder & CEO of UpScriptHealth, explores how tariffs could affect the availability and accessibility of specific medications, particularly for patients relying on direct-to-patient telehealth.

Biopharmas and global agencies are now prioritizing initiatives and innovations to drive speed across product development with an emphasis on getting new treatments in the hands of doctors and patients.

Demonstrating clinical benefit is the ultimate gateway to smoother patient access.
As part of the Inflation Reduction Act, price negotiations with manufacturers will begin this year, with reduced prices expected to take effect in 2027.

The biosimilars market in the US is on the rise, but barriers to greater adoption remain.
With the Inflation Reduction Act now in flux, how will the government and industry respond?

The importance of expanding your pharma sales forecasting horizon beyond the US. Learn how to effectively navigate country-specific regulatory red flags.
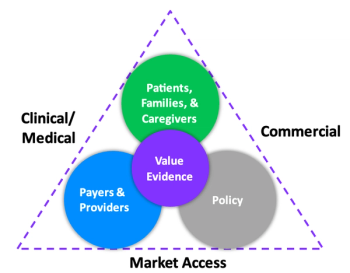
Identifying the inflators and deflators, and the role all stakeholders play.

Framework proposes three strategies designed to address the unique challenges of personalized and genetic therapies for rare diseases—and increase the probability of economic success for a new wave of potential curative treatments for these conditions.

In today’s pharmaceutical market access landscape, payers are not the sole decision-makers in utilization management development.

The Big Pharma commercialization playbook doesn’t work for emerging life sciences companies bootstrapping their way up. Exploring three strategies that could be the ingredients of a winning formula for startups.

A value deficit and potential consumer value proposition.
A new study found that among 2,200 patients with irritable bowel diseases, 63% encountered financial barriers that led to missed doses of medication.

Webinar Date/Time: Thu, Oct 17, 2024 11:00 AM EDT

The diversification of site-of-care delivery models is accelerating rapidly, creating new go-to-market implications for drug manufacturers—but also new opportunities to drive more fundamental innovation in engagement and access strategies.