
With employers and the public scrutinizing drug prices like never before, rebates-long at the center of pharma’s market access strategy-may be losing their luster, writes Amber Gilbert.

Lundbeck executive Taj Bhardwaj talks about the differing mindsets necessary to lead local and global-level market access strategy in pharma-and how his current company is committed to stay in the fight to advance new treatments and break down stigmas in mental health.

With employers and the public scrutinizing drug prices like never before, rebates-long at the center of pharma’s market access strategy-may be losing their luster, writes Amber Gilbert.

Pharm Exec’s annual feature profiling a selection of notable biopharma brands focuses this year on new beginnings and new promise-spotlighting five products with compelling product launch stories that tie strongly with the industry’s broader and evolving market-entry landscape.

Amid the noteworthy product launches profiled this year by Pharm Exec are four more therapy trailblazers worth highlighting.

Four takeaway messages from the Zolgensma pricing storm
Pharm Exec highlights a recent fireside chat between Veeva’s Matt Wallach and Jeffrey Marrazzo, CEO of Luxturna maker Spark Therapeutics.

How R&D organizations can leverage FDA’s final guidance on pre-approval information exchange (PIE) to engage with payers prior to approval and launch.

Its use, new data shows, could boost the economic benefit profile of more “common” drugs-and better inform HTA and payer decision-making.

Cell and gene therapies in crosshairs of pricing focus, prompting stepped-up proposals on ways to finance these products.

The World Health Assembly's new resolution on transparency in the market for health-related products is weaker than the original draft but still contains measures that could have a substantial impact on market access around the world, writes Neil Grubert.

While market access functions have evolved, the same can’t be said for measures used to gauge the contribution of these activities on overall business. To that end, an industry working group has developed a common framework focused on the most relevant access performance metrics (APMs).

While the increasing importance of real-world evidence (RWE) is widely acknowledged, the dramatic shift required by pharma to embed meaningful and holistic benefits from this capability is still a work in progress.

There is still no structured method of assessing pricing and access risk for drug manufacturers. Presented here is a straightforward measure for integrating pricing and access risk into portfolio planning and decision-making.

While definitions for “specialty drug” vary-and distribution and pricing/reimbursement factors remain complicated-this once-niche treatment market may be poised to steer the future of prescription medicine and patient care.
Executives highlight approaches around recent landmark approvals for digital medicine and gene therapy.

With specialty companies getting smarter in applying their big data insights to product marketing, the true commercial potential of machine learning and predictive modeling may soon be within reach.

How low-cost drugs can succeed in the specialty pharmacy channel.

There is no magic bullet that will dramatically impact drug pricing to everybody’s liking. But a stepwise approach involving a series of reforms, including taking advantage of the next midterm elections, could point the path toward a solution.
In its 12th annual feature, Pharm Exec builds on the revamped approach it introduced last year in spotlighting notable biopharma brands, profiling a new round of products that are making waves in five key areas in healthcare and R&D.

How “patient influencers” look beyond price to help the industry evaluate newly-launched brands.
Drawing from implementation lessons and successes in other industries, possible strategy shifts to pharma’s pricing playbook are explored.
Groundbreaking treatment approaches call for innovative commercialization strategies.

The five ways pharma developers can get the most out of early engagement with payers and regulators when plotting their clinical and commercial evidence plans.
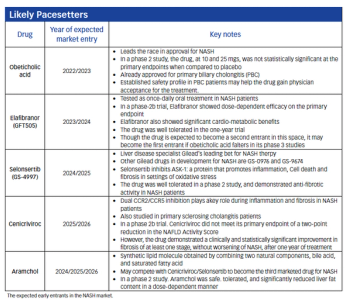
A potential rush of treatment options on the horizon for non-alcoholic steatohepatitis will require skillful maneuvering in this likely lucrative but uncharted therapeutic market.

