
ProductLife Group’s new CEO, Xavier Duburcq, talks about his hopes and ambitions, and his industry predictions, as global life sciences squares up to a more dynamic and digital future.

ProductLife Group’s new CEO, Xavier Duburcq, talks about his hopes and ambitions, and his industry predictions, as global life sciences squares up to a more dynamic and digital future.

With hundreds of clinical trials for potential coronavirus therapies in the works concerns have mounted about the emergence of conflicting data, useless results, and wasted efforts from multiple overlapping efforts.

Steve Gens and Remco Munnik offer five best-practice tips for achieving a definitive, trusted regulatory and product information asset base capable of supporting intelligent innovation.

Jill Wechsler reports on the healthcare and regulatory provisions of the $2 trillion Coronovirus Aid, Relief, and Economic Security Act.

FDA officials are rolling out guidance and support for researchers striving to assess potential treatments for COVID-19 while the agency tries to object to premature optimism and regain public credibility.

FDA is offering advice and added flexibility to help sponsors adjust ongoing and planned clinical research programs during the COVID-19 outbreak.
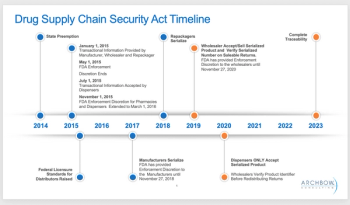
It is vital to begin asking questions about your company’s DSCSA readiness and compliance, as well as that of your strategic partners, writes Rob Besse.

Medical devices manufacturers and pharma companies alike should review their compliance strategies as the EU Medical Device Regulation Date of Application approaches.

Initiatives aim to limit the gamesmanship that can inhibit prescribing of follow-on biologics.

The Medicines and Medical Devices Bill was recently introduced to the UK House of Commons. Does it provide the confidence that the UK is still a leading country for life sciences? Cliodhna McDonough reports.

Whether or not the new Prescription Drug Pricing Reduction Act is a good policy, if it is enacted, its proposals have to work as intended and ensure compliant operationalization, writes Jeremy Docken.

New OECD study doesn’t shed much light on the effectiveness of managed entry agreements for drug access.

Weighing new strategies to accelerate biomedical product development.

New FDA commissioner Stephen Hahn announced his priorities at an “all hands” staff meeting last week. Jill Wechsler reports.

Alan White explores alternative ways for life sciences firms to keep hold of experienced, qualified professionals as demand for pharmacovigilance skills soars.

Having invested heavily in new regulatory database systems, life sciences firms owe it to themselves to capitalize on the insights locked within those rich data assets, writes David Gwyn.

Top of CDER’s to-do list for 2020 is tracking adverse events more effectively and combating the opioid crisis.

Goals for IDMP in Europe must not be diluted in 2020, if standardized medicinal product data is to be of tangible real-world benefit, says Remco Munnik.

Challenges will mount to established models for researching, developing, and marketing new therapies.

What to make of new momentum in advancing health agenda.

While not setting any records for the rapid approval of new drugs in 2019, FDA did speed a number of important new therapies to patients, writes Jill Wechsler.

Sponsors developed important new therapies amidst ongoing concerns over drug quality and costs.

While labor and tariff reforms in the revised North American trade agreement may have more visible impacts on the United States economy, the final document levels a major blow to exclusivity and patent protections important to innovator biotech and pharma companies.

Jill Wechsler looks at the CDER Office of New Drugs' new structure, which features more operational support for review functions and closer alignment of review offices to therapeutic categories.
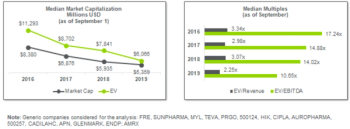
As the market for specialty and generic products continues to become more competitive and pricing pressures increase, the 505(b)(2) pathway may allow companies more options to diversify their portfolios.