
Regulatory
Latest News


FDA Approves Breyanzi for Adults with Relapsed or Refractory Marginal Zone Lymphoma
Latest Videos

More News

The agreement includes exemptions for UK-produced drugs and medical devices from Section 232 tariffs, but mandates a significant change to the UK's NICE value appraisal framework.

The agency’s decision is based on claims that the Covid vaccine was linked to the deaths of 10 children.

In the final part of this conversation, Dr. Bruce Leuchter, CEO of Neurvati Neurosciences, discusses new programs at FDA and how the neuroscience space is benefitting.

The company submitted the new dosage for approval based on its Phase III trial results.

In the first part of our conversation with Ryan Conrad, visiting fellow at the Center on Health Policy at the Brookings Institution, he provides a broad overview of the possible positive and negative impacts of FDA’s Priority Review Voucher Program.

The first wave of Commissioner’s National Priority Vouchers signals a fundamental shift in FDA competitiveness, rewarding companies that pair breakthrough science with affordability commitments, onshore manufacturing readiness, and the operational muscle to execute ultra-accelerated reviews.

A US-China Economic and Security Review Commission report highlights China’s tightening grip on APIs, biomanufacturing, and R&D services, raising alarms about supply chain vulnerabilities, data transparency gaps, and the urgent need for US policy action.
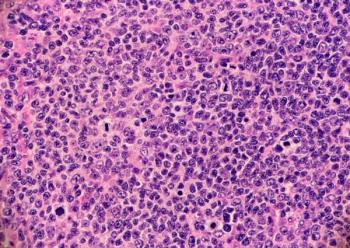
Epkinly plus rituximab and lenalidomide is the first bispecific antibody combination FDA-approved for relapsed or refractory follicular lymphoma, backed by Phase III data showing substantially improved disease control over standard therapy.

The FDA’s new Commissioner’s National Priority Voucher signals a transformative shift in U.S. drug review, tying accelerated approval to manufacturing readiness, domestic supply-chain strength, and credible affordability commitments, while redefining how companies must innovate, scale, and launch therapies in 2025 and beyond.

Approval of Komzifti (ziftomenib) provides a new targeted option for a high-risk patient population and strengthens the Kura Oncology–Kyowa Kirin collaboration, which includes a global development and commercialization strategy.

FDA introduces a new drug approval process, the Plausible Mechanism Pathway, streamlining drug approvals for personalized therapies targeting rare genetic diseases without traditional trials.
Per the FDA's request, HHS is updating hormone therapy labels, removing misleading warnings to empower women in managing menopause and improving their health options.

The FDA has added six new therapies to its Commissioner’s National Priority Voucher program, bringing the total to 15 products that address major public health needs, including obesity, cancer, sickle cell disease, and drug-resistant tuberculosis.

Johnson & Johnson secures FDA approval for Darzalex Faspro as the first and only treatment for adults with high-risk smoldering multiple myeloma, supported by Phase III Aquila trial data showing a 51% reduction in disease progression or death.

FDA’s approval of Caplyta as an adjunctive treatment to oral antidepressants for major depressive disorder in adults, expands the drug’s use to a fourth indication following positive Phase III trial results.

GLP-1 medications will be available on TrumpRx for discounted rates.

The motion is part of the state’s lawsuit against the Tylenol-maker based on unproven autism claims.

The third entry in our premium webinar series dives into the implications the MFN order might have on wider market.

FDA's approval of Kygevvi as the first treatment for thymidine kinase 2 deficiency offers hope to patients and families affected by this rare disease.

Join us for a premium webinar on November 4, 2025!

Marcel Botha, CEO of 10XBeta, discusses FDA’s new pilot authorization program for abbreviated new drug applications (ANDA) and what impact it will have on manufacturing and the supply chain.

Texas filed its lawsuit just days before Kennedy made his remarks.

Marcel Botha, CEO of 10XBeta, discusses FDA’s new pilot authorization program for abbreviated new drug applications (ANDA) and what impact it will have on manufacturing and the supply chain.

The agency says the new guidelines will simplify the requirements to bring biosimilars to market.

Marcel Botha, CEO of 10XBeta, discusses FDA’s new pilot authorization program for abbreviated new drug applications (ANDA) and what impact it will have on manufacturing and the supply chain.












