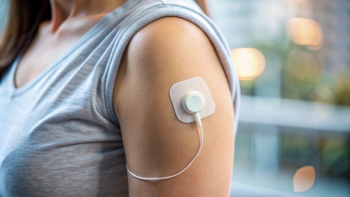
Partnership with Abbott for Simplera CGM will expand access to the advanced automated insulin delivery.

Partnership with Abbott for Simplera CGM will expand access to the advanced automated insulin delivery.
The study aims to explore Anktiva as a potential cornerstone of future immunotherapy treatments for endometrial cancer and other forms of the disease.

Voranigo is the first targeted therapy in almost 25 years to be approved by the FDA for grade 2 astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation following surgery.

The life sciences technology company will be able to offer services to the pharmacies associated with Legacy.

In an interview with Pharm Exec Associate Editor Don Tracy, Carlos Doti, VP, head of medical affairs, US oncology business unit, AstraZeneca, provides an update on current research efforts for Lynparza and discusses its impact on cancer patients over the past decade.
The FINEARTS-HF study, which compared Kerendia to a placebo when added to standard therapy, met its primary endpoint by reducing cardiovascular death and total heart failure events.
The collaboration will see both companies jointly develop and commercialize MK-6070 worldwide, except in Japan, where Merck retains exclusive rights.

A robust market access strategy ensures that pharmaceutical products, including those with limited distribution, reach patients and are covered by diverse healthcare systems.

Respondents said logistic reasons kept them from going to the doctor.
Expanded indication makes Fibryga the first and only on-demand, virus-inactivated, human plasma-derived fibrinogen concentrate approved for this indication.
The acquisition of Ypsomed’s pen needles and blood glucose monitoring system is expected to increase MTD’s production capacity to over 2.5 billion units.

The company provided guidance for how people in the storm’s path can prepare ahead of time and ensure they have access to medication.
Velys recently received 510(k) clearance from the FDA for planning and instrumenting spinal fusion procedures across the cervical, thoracolumbar, and sacroiliac spine.

The team launched from California and rowed to one of the islands of Hawaii.

The PGDx elio plasma focus Dx offers laboratories testing options for situations where samples are hard to obtain.
Regulatory action marks the first engineered cell therapy approved for a solid tumor cancer in the United States.

Approval marks the first time an immuno-oncology regimen has demonstrated a survival benefit for all adults with primary advanced or recurrent endometrial cancer, company says.
IDeate-Lung02 will compare ifinatamab deruxtecan to a physician’s choice of chemotherapy in patients with relapsed small cell lung cancer.

Data from the SUMMIT clinical trial demonstrated that tirzepatide lowered the risk of negative heart failure outcomes and enhanced symptoms and physical limitations when tested with three different doses.

In an interview with Pharm Exec Associate Editor Don Tracy, Carlos Doti, VP, head of medical affairs, US oncology business unit, offers his thoughts on the evolution of Lynparza across indications such as breast, prostate, and pancreatic cancers.

Results of the Phase III Clarity AD study found that Leqembi reduced cognitive decline by -0.95 on the Clinical Dementia Rating-Sum of Boxes compared to expected declines in untreated groups.
Results from the PERSEUS study led to the FDA approval of Darzalex Faspro in patients with newly diagnosed multiple myeloma who are eligible for an autologous stem cell transplant.
With a growing number of legal battles over women’s health issues, such as abortion and infertility, stakeholders are concerned about the impact on everything from healthcare access to new technology.

The report also details steps that employers are taking to manage the cost of healthcare plans for employees.

The latest news for pharma industry insiders.

Erzofri is the first patented paliperidone palmitate long-acting injectable developed in China to receive FDA approval for treating schizophrenia and schizoaffective disorder, company says.
Results from the ASC4FIRST study lead to FDA priority review designation of Scemblix in newly diagnosed patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase.

The company announced that it will conduct research based on guidance from the agency.

Ouva’s AI-powered technology will be added to AvaSure’s digital platform.
Approval of Zunveyl offers a novel approach with a dual mechanism of action designed to improve tolerability and efficacy in treating Alzheimer disease, company says.